Method for recovering copper from acidic waste water containing copper
A technology for recovering copper and wastewater, applied in crucible furnaces, electric furnaces, drum furnaces, etc., can solve the problems of inability to obtain high-purity copper, poor copper purity, and difficult separation, and achieve shortened recovery cycle, low recovery cost, The effect of speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
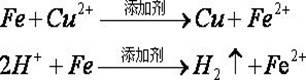
Image
Examples
Embodiment 1
[0017] Add 2 tons of acidic copper-containing wastewater into the acid-resistant reaction tank, measure the concentration of copper ions in the solution to 26.45g / L, and calculate the amount of iron required. Add a small amount of dilute sulfuric acid to adjust the pH value of the solution to 0.5, add 50g of additives and stir evenly, then throw 90kg of scrap iron nets into the copper-containing wastewater at one time and immerse in the solution. When the reaction was carried out for about 2 hours, the pH value of the whole solution reached about 6. At this time, the remaining copper ion concentration in the solution was only 16.32mg / L, and more than 99% of the copper in the solution was replaced. The net is stirred in the solution for a few times, and then it is taken out. There is no copper attached to the iron net. The recovered waste liquid is discharged into the acid waste water collection tank, and then the copper powder at the bottom of the tank is taken out, washed with...
Embodiment 2
[0019] Add 2 tons of acidic copper-containing wastewater into the acid-resistant reaction tank, measure the concentration of copper ions in the solution to 31.42g / L, and calculate the amount of iron required. Add a small amount of dilute sulfuric acid to adjust the pH value of the solution to 1.0, add 30g of additives and stir evenly, then throw 166 kg of scrap iron nets into the copper-containing wastewater at one time and immerse in the solution. When the reaction was carried out for about 2 hours, the pH value of the whole solution reached about 6. At this time, the remaining copper ion concentration in the solution was only 16.53mg / L, and more than 99% of the copper in the solution was replaced. The net is stirred in the solution for a few times, and then it is taken out. There is no copper attached to the iron net. The recovered waste liquid is discharged into the acid waste water collection tank, and then the copper powder at the bottom of the tank is taken out, washed wi...
Embodiment 3
[0021] Add 5 tons of acidic copper-containing wastewater into the acid-resistant reaction tank, measure the concentration of copper ions in the solution to 21.26g / L, and calculate the amount of iron required. Add a small amount of dilute sulfuric acid to adjust the pH value of the solution to 1.5, add 100g of additives and stir evenly, then throw 166 kg of scrap iron nets into the copper-containing wastewater at one time and immerse in the solution. When the reaction was carried out for about 2.5 hours, the pH value of the whole solution reached about 6. At this time, the remaining copper ion concentration in the solution was only 21.58mg / L, and more than 99% of the copper in the solution was replaced. The iron net is stirred in the solution for a few times, and then taken out. There is no copper attached to the iron net. The recovered waste liquid is discharged into the acid waste water collection tank, and then the copper powder at the bottom of the tank is taken out, washed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com