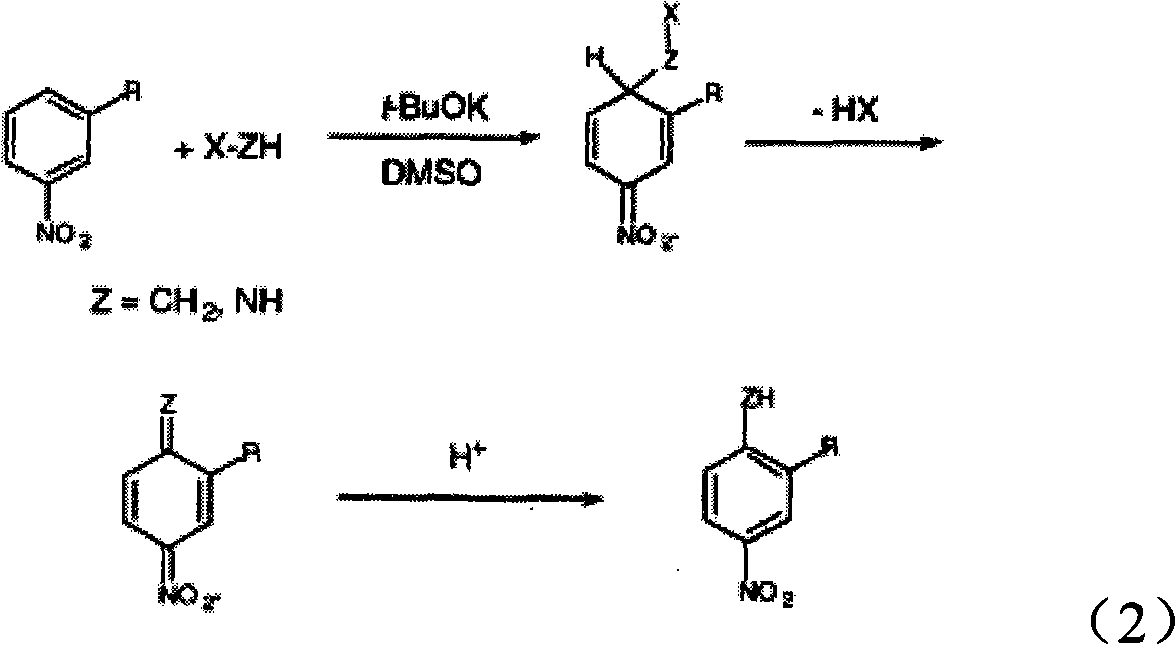
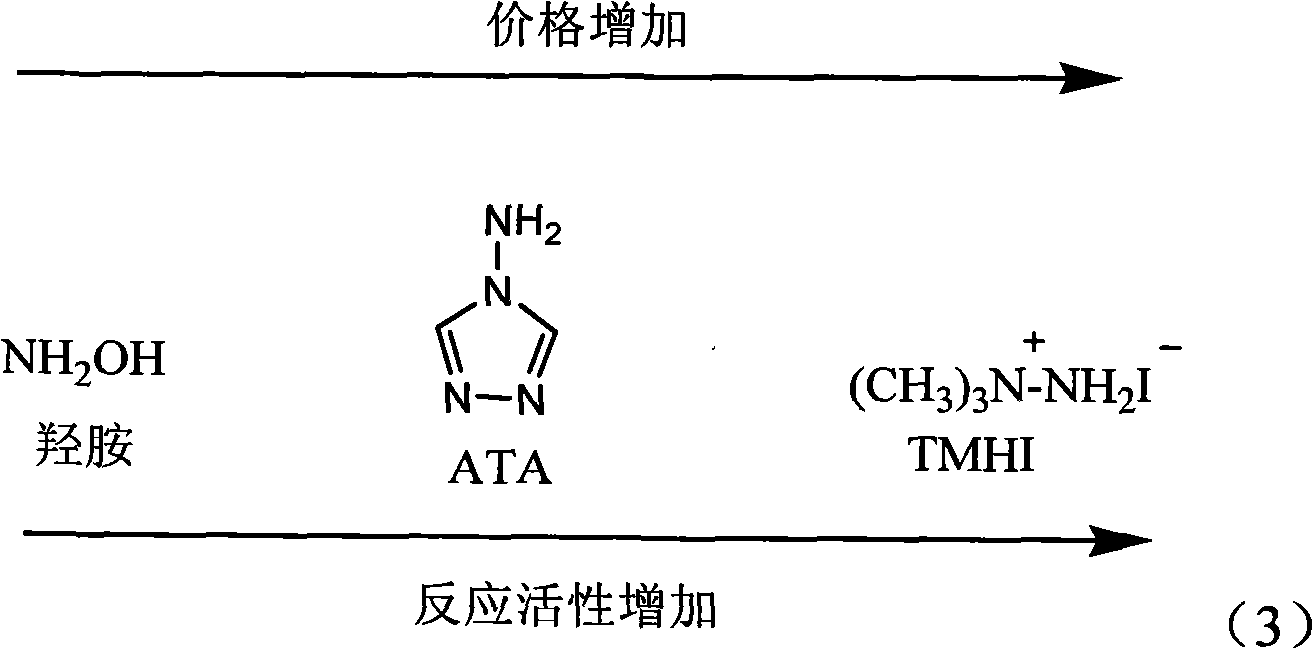
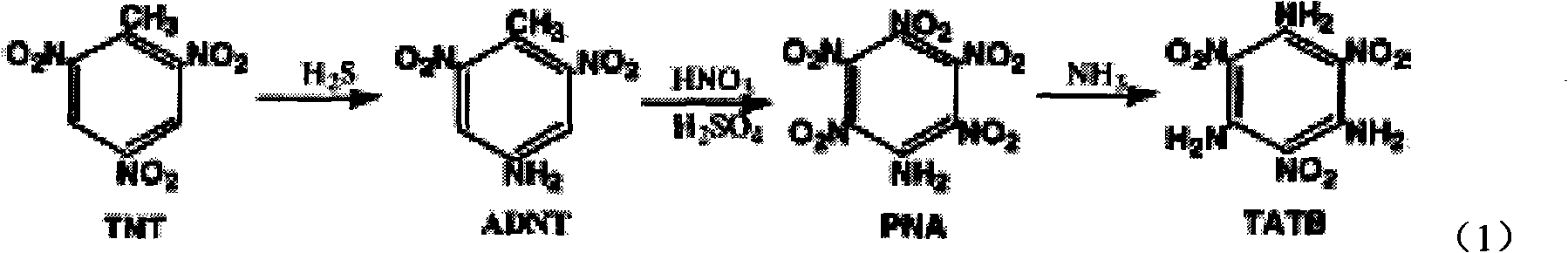Method for synthesizing tri-amino trinitrobenzene (TATB) by trinitrotoluene (TNT)
A high-yield, concentrated nitric acid technology, applied in the preparation of amino groups replacing hydrogen atoms, organic chemistry, etc., can solve the problems of demanding reaction equipment, achieve the effects of optimizing kinetic conditions, reducing costs, and avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Dissolve 1g of TNT in 1.5mL of concentrated nitric acid, heat to 110°C, then add 1g of saturated aqueous solution of sodium chlorate, react for 3h, cool to room temperature, add ice water, filter to obtain 1.02g of trinitrobenzoic acid TNBA; Yield is 90%;
[0032] 2) Add 5mL of water to the TNBA obtained in step 1), add 1mL of 20% sodium hydroxide aqueous solution under stirring, filter, heat the filtrate to 100°C for decarboxylation reaction, the heating time is 3h, stop heating after the reaction is completed, and generate trinitrate Phenylbenzene TNB solid was suspended in the solution, cooled, filtered, and dried to obtain 0.72g TNB solid with a yield of 85%;
[0033] 3) Under nitrogen protection, mix 3mmol ATA, 6mmol sodium methoxide and 3mL DMSO to form a suspension, stir for 20min; then add 2mL of DMSO dissolved with 0.21gTNB into the suspension, heat up to 50°C, react for 6h, and the reaction is complete After cooling, pour it into 2001 mL of distilled water...
Embodiment 2
[0035] Same as Example 1, except that DMSO is DMF in step 3), and finally 0.248gTATB is obtained with a yield of 98%.
Embodiment 3
[0037] Same as Example 1, but the difference is that DMSO is THF in step 3), and finally 0.24g TATB is obtained with a yield of 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com