Method for preparing anti-hyperosteogeny tablets
A technology of anti-bone hyperplasia and rhizoma drynaria, which is applied in the field of medicine, can solve the problems that it is difficult to cover up the bad taste of drugs, the time limit for dissolving pills is difficult to control, and the bad smell of drugs is not easy to cover up, and achieves good dissolution rate and bioavailability. The effect of small content difference and accurate dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Take 204g rehmannia glutinosa, 136g chrysanthemum chrysalis, 136g rhizoma drynaria (hot), 136g Caulis Spatholobus, 136g cistanche, 136g epimedium, radish seed (stir-fried) 86g by weight;
[0032] (2) Get half of the amount of Miltonia Spatholobus described in step (1) and pulverize, then pass through a 100-mesh sieve;
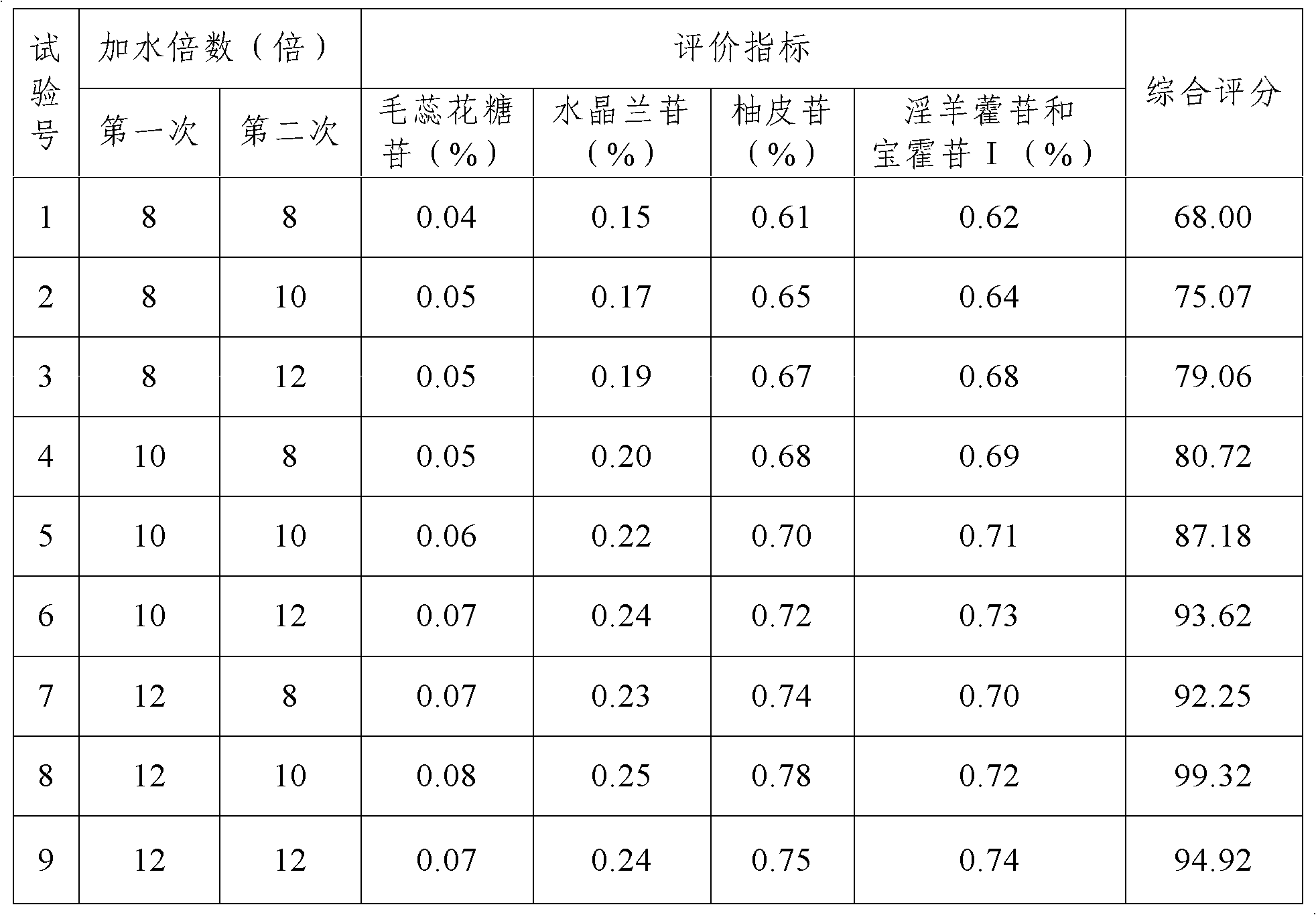
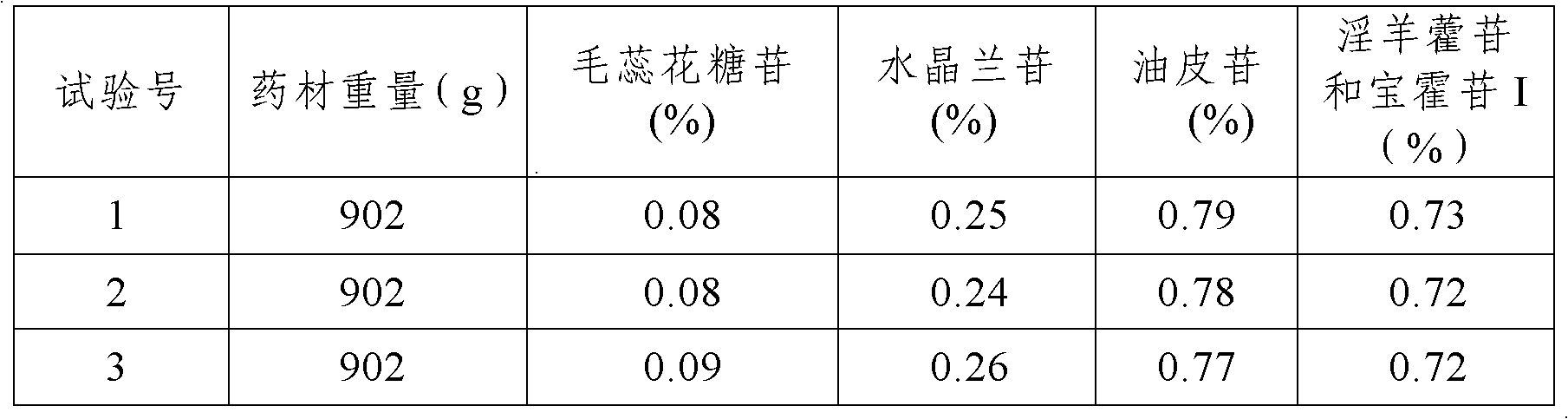
[0033] (3) Boil Rehmannia glutinosa, Luniancao, Drynaria officinalis (hot), Cistanche, Epimedium, Laifuzi (fried), and the other half of the seven herbs of Spatholobus Spatholobus twice. The amount of water is 12 times of the total weight of the medicinal materials to be decocted, the decoction time is 3 hours, the amount of water added for the second decoction is 10 times of the total weight of the medicinal materials to be decocted, the decoction time is 2 hours, and the filtrate is combined after filtration. Concentrate to a thick paste with a relative density of 1.30 (50°C);
[0034] (4) Add the sifted Caulis Spatholobus in step (2) to the thick...
Embodiment 2
[0036] The preparation method of this example is the same as that of Example 1, except that the raw materials used are 254g of rehmannia glutinosa, 186g of Herba Ceratum, 186g of Rhizoma Drynariae (hot), 186g of Caulis Spatholobus, 186g of Herba Cistanche, and 186g of Epimedium 186g, radish seed (fried) 186g.
Embodiment 3
[0038]The preparation method of this example is the same as that of Example 1, except that the raw materials used are 154g Rehmannia glutinosa, 86g Rhizoma cerevisiae, 86g Rhizoma Drynariae (hot), 86g Caulis Spatholobus, 86g Cistanche, Epimedium 86g, radish seed (fried) 36g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com