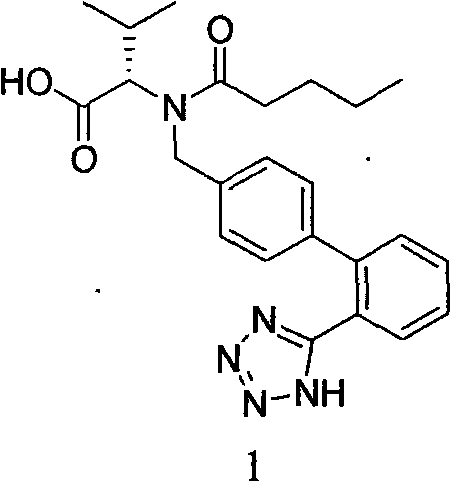
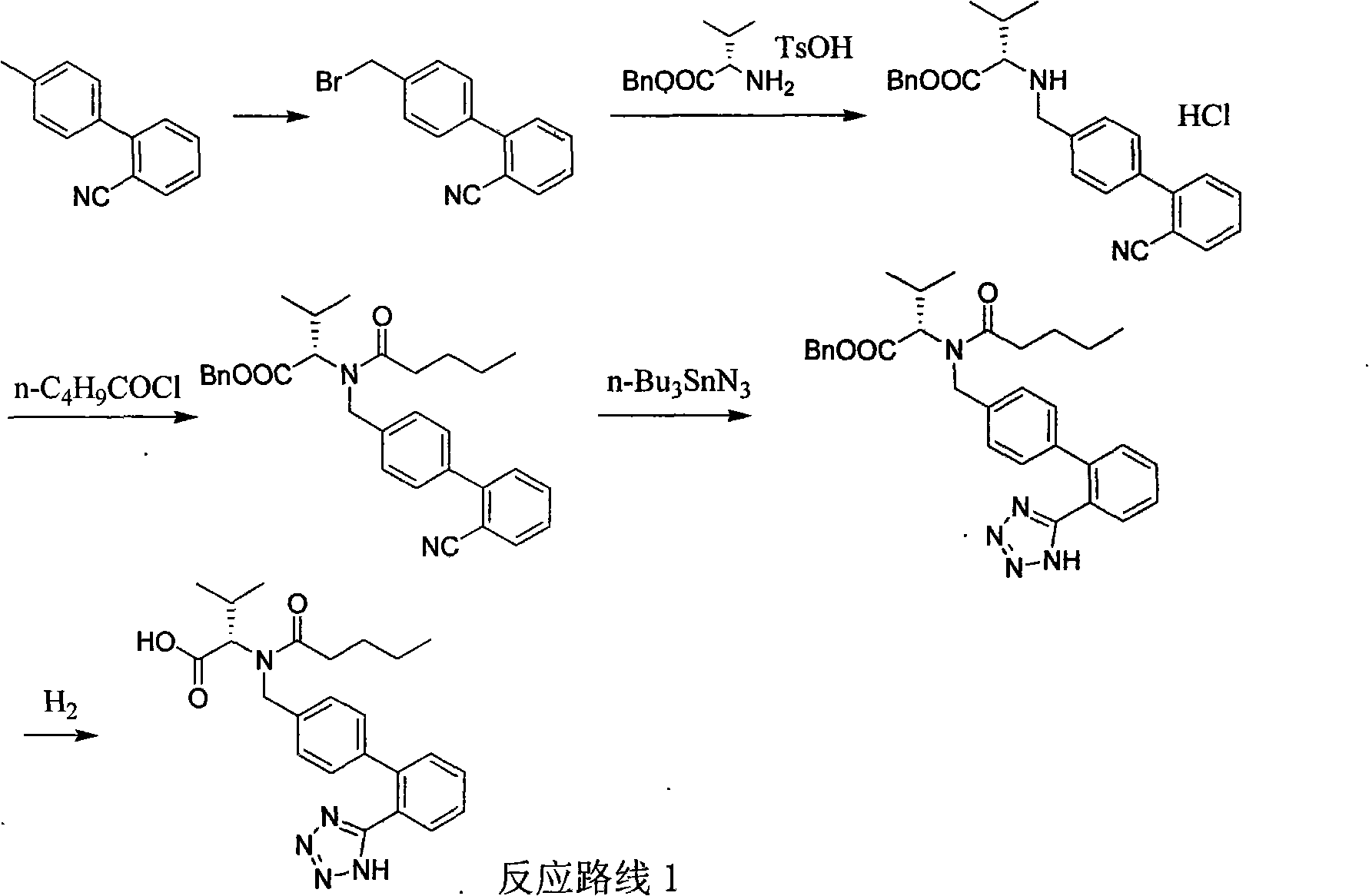
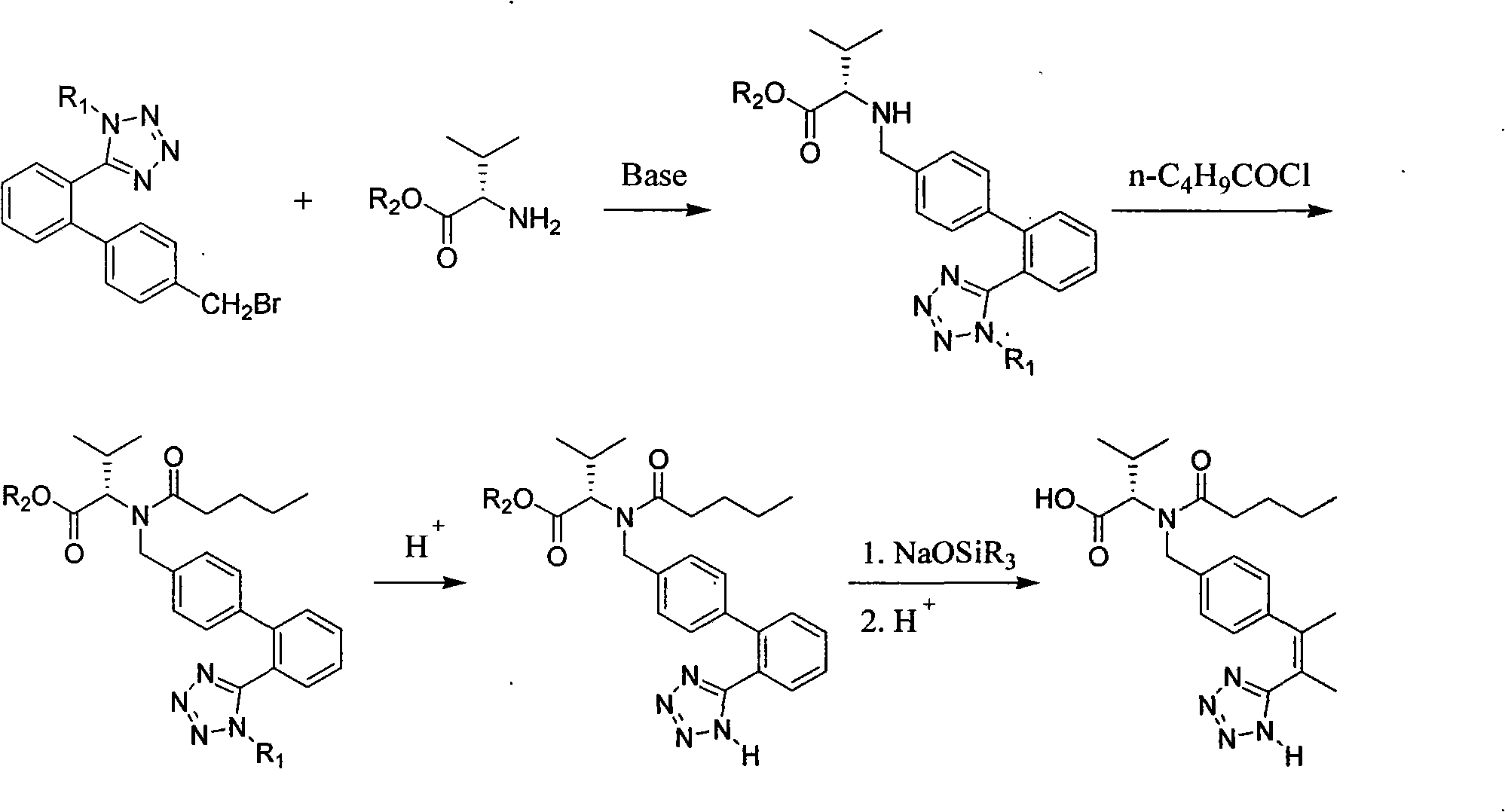
Improved preparation method of valsartan
A technology of valsartan and tetrazolium ring, applied in the field of synthesizing valsartan, can solve the problems of high price, high reflux temperature, toxicity and the like, and achieve the effects of simplifying operation, improving yield and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1. Azidotrimethylsilane (Me 3 SiN 3 ) preparation
[0028] Add trimethylchlorosilane (Me 3 SiCl, 66.6g, 0.616mol), sodium azide (NaN 3 , 48.0g, 0.739mol), n-butyl ether (300mL), heated to 100°C for 48 hours, distilled, and collected the fraction at 90°C-105°C, which was the crude product of azidotrimethylsilane, and the crude product was redistilled 56.7 g of colorless liquid was obtained, yield 80.0%, boiling point: 95°C-96°C.
Embodiment 2
[0029] Example 2. Azidotrimethylsilane (Me 3 SiN 3 ) preparation
[0030] In a 500mL four-neck reaction flask equipped with a reflux condenser, a thermometer and a magnetic stirrer, add silicone oil (150mL), sodium azide (NaN 3 , 38.5g, 0.592mol) and polyethylene glycol (1.3g), the mixture was heated to 50°C-59°C, and trimethylchlorosilane (Me 3 SiCl, 64.3g, 0.592mol), after the dropwise addition, keep the reaction at 50°C-59°C for 2 hours. After the reaction is completed, heat the reaction solution to 105°C and distill to obtain azidotrimethylsilane, which is colorless Liquid 62.4g, yield 91.6%, boiling point: 95°C-96°C.
Embodiment 3
[0031] Example 3. N-(1-oxopentyl)-N-[[2'-(1H-tetrazol-5-yl)-(1,1'-diphenyl)-4-yl]-methanol Preparation of base]-L-valine methyl ester (II)
[0032] Add tetrabutylammonium fluoride trihydrate (TBAF.3H 2 O, 0.78g, 2.46mmol), azidotrimethylsilane (Me 3 SiN 3 , 5.67g, 49.3mmol, prepared in Example 1), N-(1-oxopentyl)-N-[[2'-cyano-(1,1'-diphenyl)-4-yl]- Methyl]-L-valine methyl ester (1, 10g, 24.6mmol), the mixture was vigorously stirred, and reacted at 80°C for 50 hours, TLC (petroleum ether: ethyl acetate=3:1) detected that the reaction was complete, and the reaction Cool the liquid to room temperature, add xylene (100mL) and water (20mL), stir for 30min, separate layers, wash the organic phase with 1mol / L HCl (25mL×3) to remove tributylammonium fluoride, and dry over anhydrous sodium sulfate N-(1-oxypentyl)-N-[[2'-cyano-(1,1'-diphenyl)-4-yl]-methyl]-L-valine methyl ester (II ) xylene solution, directly used for the next step hydrolysis reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com