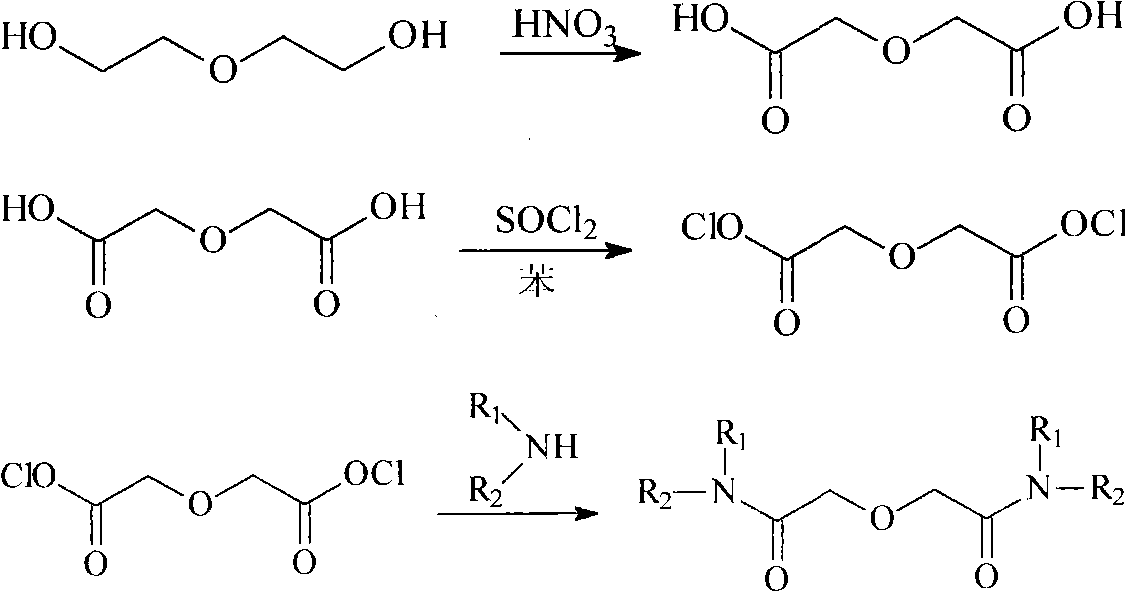
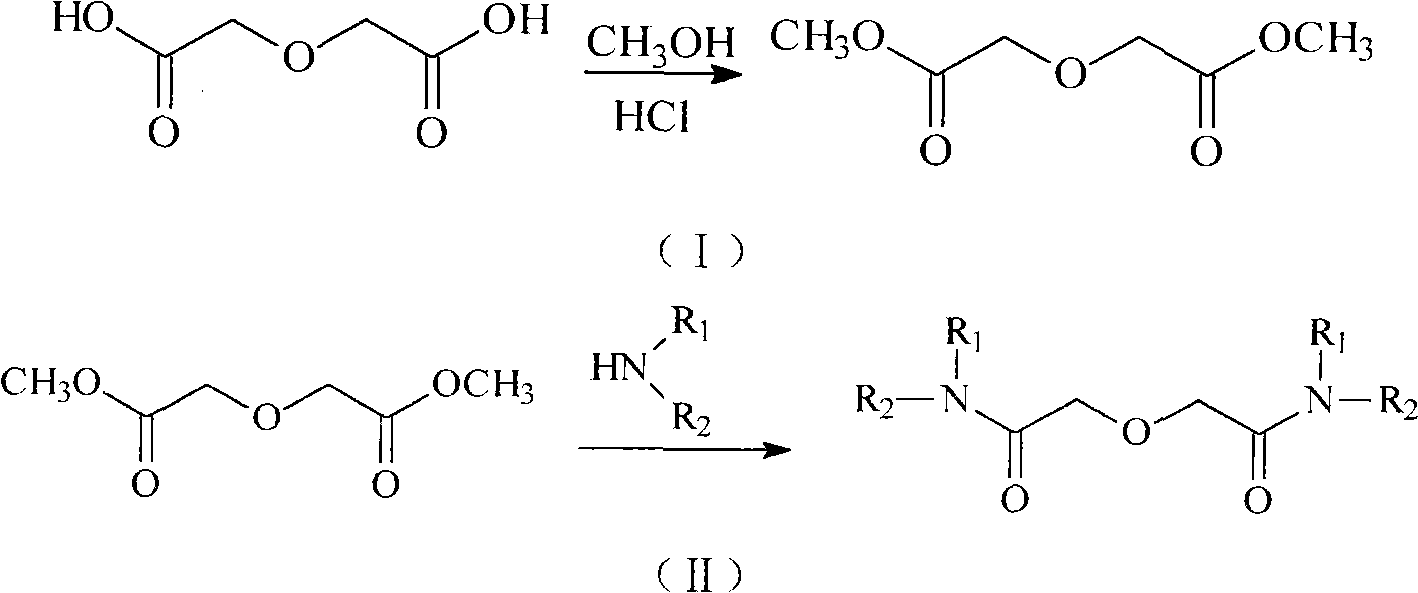
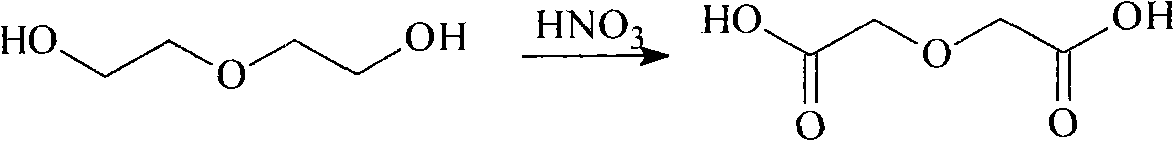Method for synthesizing diamide compound (R1R2NCO) 2CH2OCH2
A 2CH2OCH2, synthetic method technology, applied in the preparation of organic compounds, chemical instruments and methods, preparation of carboxylic acid amides, etc., can solve the problems of large amount of instrument usage, high cost, large amount of waste gas and waste liquid, etc. The effect of low pollution and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1N, N, N', the preparation of N'-tetrabutyl-3-oxoglutaramide (TBOPDA)
[0019] 1 Preparation of Diglycolic Acid
[0020]
[0021] Add 1000g of concentrated nitric acid (about 714mL) into a 1000mL three-flask equipped with a constant pressure funnel, a thermometer and a reflux device, heat under magnetic stirring until the temperature in the flask reaches 65°C, stop heating, and drop a few drops of absolute ethanol as a trigger At this time, reddish-brown gas was released in the flask, and 1mol (106mL) diethylene glycol was added under stirring, and the rate of addition was controlled to keep the reaction temperature between 65-70°C. The reaction was violent, and a large amount of reddish-brown gas was released. Absorb with 5% sodium hydroxide solution. After the dropwise addition was completed, the temperature was raised to 80°C until the nitrogen dioxide in the bottle was no longer generated, and 200 mL of distilled water was added to stop the reaction. ...
Embodiment 2
[0033] Embodiment 2: N, N'-dimethyl-N, the preparation of N'-diphenyl-3-oxyglutaramide (DMDPhOPDA)
[0034] The preparation of diglycolic acid and dimethyl diglycidate is the same as in Example 1.
[0035] N, N'-dimethyl-N, the preparation reaction formula of N'-diphenyl-3-oxyglutaramide (DMDPhOPDA) is:
[0036]
[0037] Add 32.43g dimethyl diglycidate (0.2mol, 162.14g mol-1) and 53.5g N-methylaniline (0.5mol, 107.15g mol-1) into a three-necked flask, with a magnetic stirrer and oil bath, top-mounted thermometer and reflux condenser. Slowly heat to a temperature of about 50°C, all the solids in the flask melt, start stirring, and control the temperature at 160°C for reaction. The reaction was stopped after the completion of the reaction detected by thin-layer chromatography, and it took about 4-6 hours.
[0038] After the reaction is completed, filter and distill off methanol and unreacted N-methylaniline under reduced pressure. After diluting the remaining substance wit...
Embodiment 3
[0039] Embodiment 3: N, N'-dimethyl-N, the preparation of N'-diphenyl-3-oxyglutaramide (DMDPhOPDA), used N-methylaniline gets 0.4mol, other is with embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com