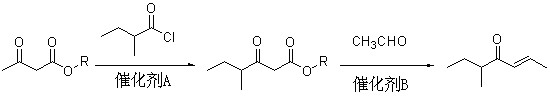
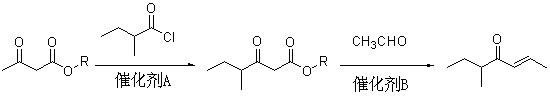
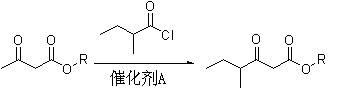Method for preparing 5-methyl-2-hepten-4-one
A technology of heptene and methyl, which is applied in the field of preparation of 5-methyl-2-hepten-4-one, can solve the problems of difficult industrial production, cumbersome operation, and large pollution, and achieve easy industrial production and simple operation , the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1), the preparation of ethyl 2-methylbutyroacetate
[0037] In a 2L four-neck flask equipped with mechanical stirring, dropping funnel and thermometer, add 77.8g (1.05mol) of calcium hydroxide and 500ml of dichloromethane. Under rapid stirring, 130 g (1.0 mol) of ethyl acetoacetate was slowly added dropwise, the temperature was controlled at 20-30°C, and the dropwise addition time was about 20 minutes. After the dropwise addition, continue to stir for 0.5 hours, then add 138.6g (1.15mol) of 2-methylbutyryl chloride dropwise to it, maintain the temperature at 30-35°C, drop the time for about 1.5 hours, and then continue at 40°C Stir for 2 hours;
[0038] Dissolve 56.2g (1.05mol) of ammonium chloride in 350ml of water, add it to the above reactant at 30°C, stir for half an hour, add an appropriate amount of ammonia water to adjust the pH value of the solution to 9, and then add it at 30-35 Continue to stir for 3 hours at ℃ to obtain a solution containing ethyl 2-methyl...
Embodiment 2
[0045] (1), the preparation of ethyl 2-methylbutyroacetate
[0046] In a 2L four-neck flask equipped with mechanical stirring, dropping funnel and thermometer, add 77.8g (1.05mol) of calcium hydroxide and 500ml of dichloromethane. Under rapid stirring, 130 g (1.0 mol) of ethyl acetoacetate was slowly added dropwise, the temperature was controlled at 20-30°C, and the dropwise addition time was about 20 minutes. After the dropwise addition, continue to stir for 0.5 hours, then add 132.5g (1.10mol) of 2-methylbutyryl chloride dropwise to it, maintain the temperature at 30-35°C, drop the time for about 1.5 hours, and then continue at 40°C Stir for 2 hours;
[0047] Dissolve 56.2g (1.05mol) of ammonium chloride in 350ml of water, add it to the above reactant at 30°C, stir for half an hour, add an appropriate amount of ammonia water to adjust the pH value of the solution to 9, and then add it at 30-35 Continue to stir for 3 hours at ℃ to obtain a solution containing ethyl 2-methyl...
Embodiment 3
[0054] (1), the preparation of ethyl 2-methylbutyroacetate
[0055] In a 2L four-neck flask equipped with mechanical stirring, dropping funnel and thermometer, add 77.8g (1.05mol) of calcium hydroxide and 500ml of dichloromethane. Under rapid stirring, 130 g (1.0 mol) of ethyl acetoacetate was slowly added dropwise, the temperature was controlled at 20-30°C, and the dropwise addition time was about 20 minutes. After the dropwise addition, continue to stir for 0.5 hours, then add 126.5g (1.05mol) of 2-methylbutyryl chloride dropwise to it, maintain the temperature at 30-35°C, drop the time for about 1.5 hours, and then continue at 40°C Stir for 2 hours;
[0056] Dissolve 56.2g (1.05mol) of ammonium chloride in 350ml of water, add it to the above reactant at 30°C, stir for half an hour, add an appropriate amount of ammonia water to adjust the pH value of the solution to 9, and then add it at 30-35 Continue to stir for 3 hours at ℃ to obtain a solution containing ethyl 2-methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com