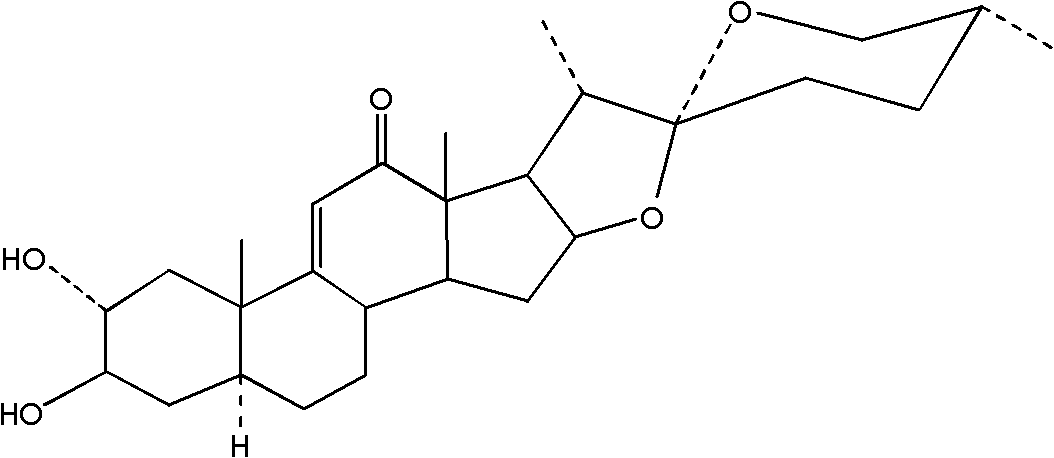
Method for preparing high purity 9,11-dehydromanogenin
A high-purity hydromannogenin technology is applied in the preparation of 9 fields with a mass percentage purity greater than 95%, which can solve the problems of difficulty in mass preparation, cumbersome operation, low yield, etc., and achieves reduction in dosage, simplification of processes, and cost reduction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] This embodiment 1 is implemented under the following conditions of implementation and technical requirements:
[0026] 1) 500g of the rhizome of Calyx aurantiae, crushed into a coarse powder, add 2500mL of 70(v / v)% industrial ethanol to reflux for extraction for 2h, filter, recover ethanol from the filtrate (for the next extraction), and obtain extract; repeat the above operation 1 time, combined 2 times of the gained extract to obtain alcoholic extract 117g;
[0027] 2) Take the alcohol extract, add 300mL of water to suspend, then extract 3 times with 300mL of dichloromethane, combine the dichloromethane layers, and recover the dichloromethane under reduced pressure (for the next extraction);
[0028] 3) The water layer after dichloromethane extraction was continued to be extracted 3 times with 300 mL of water-saturated n-butanol, the n-butanol layers were combined, and n-butanol was recovered (for the next extraction), to obtain 42 g of total saponins;
[0029] 4) Di...
Embodiment 2
[0032] This embodiment 2 is implemented under the following conditions of implementation and technical requirements:
[0033] 1) 500 g of purple calyx leaves, crushed into coarse powder, added 4000 mL of 95 (v / v)% industrial ethanol for reflux extraction for 2 hours, filtered, recovered ethanol from the filtrate (for the next extraction) to obtain extract. Repeat the above operation, combine the extracted extract obtained twice to obtain 159g of alcoholic extract;
[0034] 2) Take the alcohol extract, add 350mL of water to suspend, then extract 3 times with 350mL of dichloromethane, combine the dichloromethane layers, and recover the dichloromethane (for the next extraction);
[0035] 3) The water layer after dichloromethane extraction was continued to be extracted 3 times with 350 mL of water-saturated n-butanol, the n-butanol layer was combined, and n-butanol was recovered (for the next extraction) to obtain 36 g of total saponins;
[0036] 4) Dissolve the total saponins in...
Embodiment 3
[0039] This embodiment 3 is implemented under the following conditions of implementation and technical requirements:
[0040] 1) 500g of the whole plant of Violet Calyx, crushed into coarse powder, added 3500mL of 80(v / v)% industrial ethanol to reflux for extraction for 2h, filtered, recovered ethanol from the filtrate (for the next extraction) to obtain extract. The above operation was repeated once, and the extracted extracts obtained from the two extractions were combined to obtain 120 g of alcoholic extracts.
[0041] 2) Take the alcohol extract, add 300mL of water to suspend, then extract 3 times with 300mL of dichloromethane, combine the dichloromethane layers, and recover the dichloromethane under reduced pressure (for the next extraction).
[0042] 3) The water layer after dichloromethane extraction was continued to be extracted three times with 300 mL of water-saturated n-butanol, the n-butanol layers were combined, and n-butanol was recovered (for the next extraction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com