Monoclonal antibody of hepatitis B virus X protein and use thereof
A monoclonal antibody and protein technology, applied in antiviral immunoglobulins, antibodies, antiviral agents, etc., can solve the problems of low efficiency of monoclonal antibodies and weak immunogenicity of HBx
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
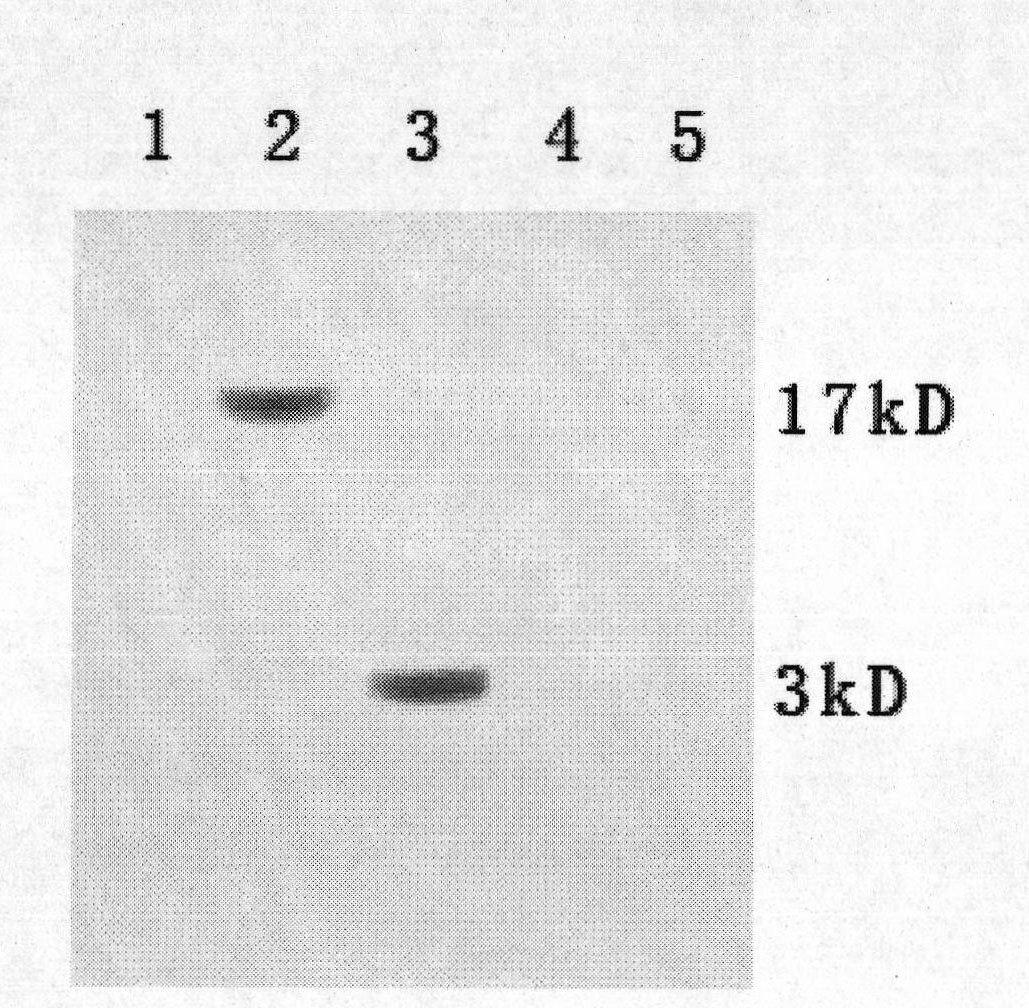
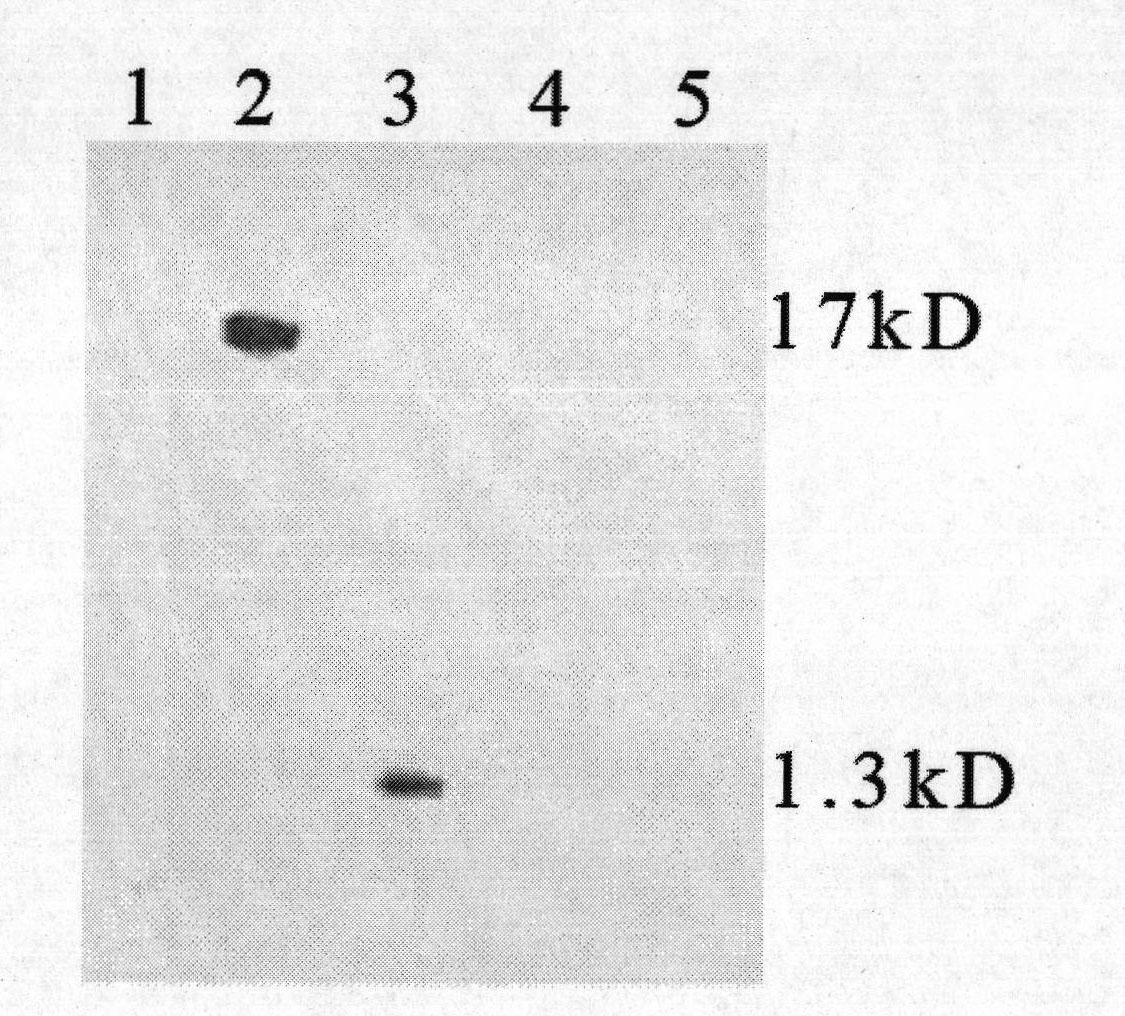
[0033] Example 1, Screening of HBx Monoclonal Antibody
[0034] 1. Synthesis of HBx N-terminal and C-terminal antigenic epitope polypeptides: Antigenic Peptides antigenic epitope analysis online software was used to analyze the antigenic epitope of the amino acid sequence of HBx protein. The results showed that the HBx protein N-terminal 11-20 amino acid sequence PARDVLCLRP and the HBx protein C-terminal 127-150 amino acid sequence IRLKVFVLGGCRHKLVCSPAPCNF were segments with higher epitope index. The HBx N-terminal and C-terminal antigenic epitope polypeptides shown in polypeptide sequence 1 (SEQ 1) and polypeptide sequence 2 (SEQ 2) were synthesized by artificial or chemical synthesis method respectively.
[0035] 2. Cross-linking of HBx N-terminal or C-terminal antigenic epitope polypeptide and carrier protein keyhole limpet hemocyanin (KLH): dissolving maleimide-activated keyhole limpet hemocyanin (KLH) maKLH in water (Themo Scientific company product), so that the concent...
example 2
[0039] Example 2, Preparation, purification and identification of HBx monoclonal antibody
[0040]1. Preparation and purification of HBx monoclonal antibody: For large-scale preparation of monoclonal antibody, hybridoma cells were inoculated into the peritoneal cavity of mice, and the ascites of mice was collected to obtain antibodies. Antibody purification can be carried out by methods such as salting out, gel filtration, and affinity chromatography. We prefer the ammonium sulfate-octanoate precipitation method for purification. (1) Preparation of ascites: first intraperitoneally inject 0.5ml Pristane (pristane) or liquid paraffin into BaLb / c mice, and then intraperitoneally inject 1×10 6 Ascites can be produced 7 to 10 days after the inoculation of cells. Closely observe the health status of the animals and signs of ascites. When the ascites is as much as possible, and the mice die frequently, the mice are killed and the ascites is collected. (2) Purification of monoclonal...
example 3
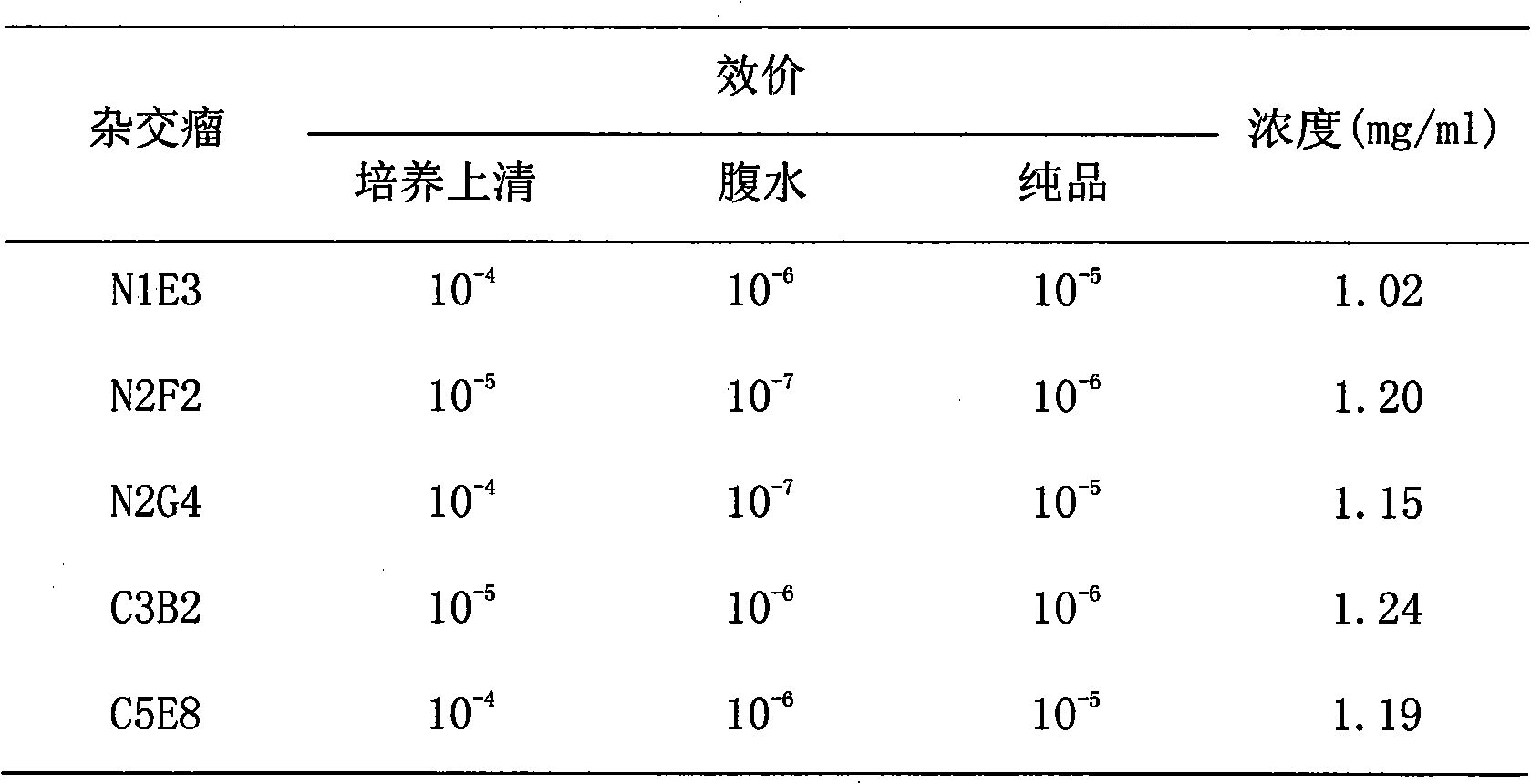
[0046] Example three, HBx monoclonal antibody is used to prepare HBx protein enhanced chemiluminescence immunoassay kit
[0047] 1. HBx N-terminal and C-terminal epitope-specific monoclonal antibody pairing experiment: HBx N-terminal epitope-specific monoclonal antibodies N1E3, N2F2, N2G4 and HBx C-terminal epitope-specific monoclonal antibodies C3B2, C5E8 Perform reciprocal pairing experiments. Experiment 1: Use 50 μl of 20 μg / ml N-terminal epitope-specific monoclonal antibodies N1E3, N2F2 and N2G4 as capture antibodies to coat microwell plates, and the coating buffer is carbonate buffer (PH9.5). HRP-labeled HBx C-terminal epitope-specific monoclonal antibodies C3B2 and C5E8 were used as detection antibodies, adding serially diluted recombinant HBx protein and HBx positive quality control serum, washing with washing solution 3 times to wash off non-specific binding antigens, adding HRP-labeled 1E34 and 3B23 monoclonal antibodies were used for paired comparison experiments, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com