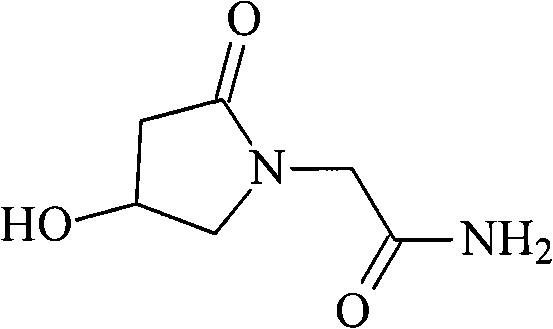
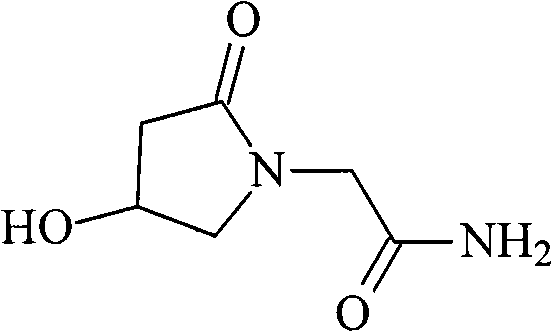
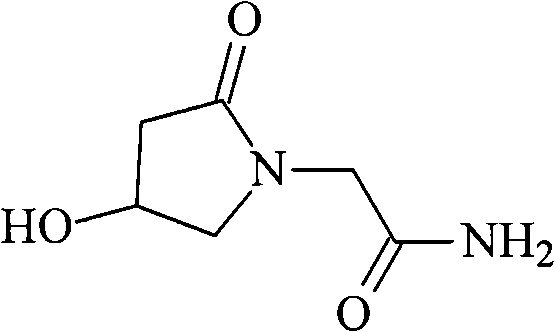
Oxiracetam compound and new method thereof
A compound, separation and purification technology, applied in the medical field, can solve the problems of insufficient purity, no public purification of oxiracetam compounds, etc., to achieve the effect of improving purity and content, simple and easy process, and ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 The refining of oxiracetam
[0038] (1) Dissolve 100 g of crude oxiracetam with a purity of 96.7% in 1000 ml of purified water, add 2 g of activated carbon, stir at 60° C. for 20 min, filter for decarburization, and collect the filtrate;
[0039] (2) in gained filtrate, slowly add the mixed solvent 3000ml that volume ratio is the acetonitrile and isopropyl ether of 2: 1, stir, produce precipitation, filter and obtain oxiracetam solid;
[0040] (3) put oxiracetam solid on the column, separate and purify by chromatographic column, with the acetonitrile and water mixed solvent of volume ratio 1: 3 as mobile phase, stationary phase filler is aluminum oxide, flow velocity is 3.0ml / min, column temperature 30°C, collect the fractions in sections, and combine the eluents with a content greater than 90%;
[0041] (4) Concentrate the eluent under reduced pressure to about 1 / 2 volume, add 1000ml of acetonitrile and isopropyl ether mixed solvent with a volume ratio of 2:...
Embodiment 2
[0042] Example 2 The refining of oxiracetam
[0043] (1) Dissolve 100 g of crude oxiracetam with a purity of 96.7% in 1000 ml of purified water, add 5 g of activated carbon, stir at 50° C. for 30 min, filter for decarburization, and collect the filtrate;
[0044] (2) in gained filtrate, slowly add the mixed solvent 6000ml that volume ratio is 2: 1 acetonitrile and isopropyl ether, stir, produce precipitation, filter to obtain oxiracetam solid;
[0045] (3) put oxiracetam solid on the column, separate and purify by chromatographic column, be mobile phase with the mixed solvent of acetonitrile and water of 1: 3 with the volume ratio, stationary phase filler is aluminum oxide, flow velocity is 5.0ml / min, column Warm at 40°C, collect the fractions in sections, and combine the eluents with a content greater than 90%;
[0046] (4) Concentrate the eluent under reduced pressure to about 1 / 2 volume, add 1000 ml of acetonitrile and isopropyl ether mixed solvent with a volume ratio of 2...
Embodiment 3
[0047] Example 3 The purification of oxiracetam
[0048] (1) Dissolve 100 g of crude oxiracetam with a purity of 96.7% in 1000 ml of purified water, add 3 g of activated carbon, stir at 60° C. for 30 min, filter for decarburization, and collect the filtrate;
[0049] (2) in the obtained filtrate, slowly add the mixed solvent 4000ml of acetonitrile and isopropyl ether with a volume ratio of 2: 1, stir to produce precipitation, filter to obtain the oxiracetam solid;
[0050] (3) put oxiracetam solid on the column, separate and purify by chromatographic column, be mobile phase with the mixed solvent of acetonitrile and water of 1: 3 with the volume ratio, stationary phase filler is aluminum oxide, flow velocity is 2.5ml / min, column Warm at 25°C, collect the fractions in sections, and combine the eluents with a content greater than 90%;
[0051] (4) Concentrate the eluent under reduced pressure to about 1 / 2 volume, add 1000 ml of acetonitrile and isopropyl ether mixed solvent wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com