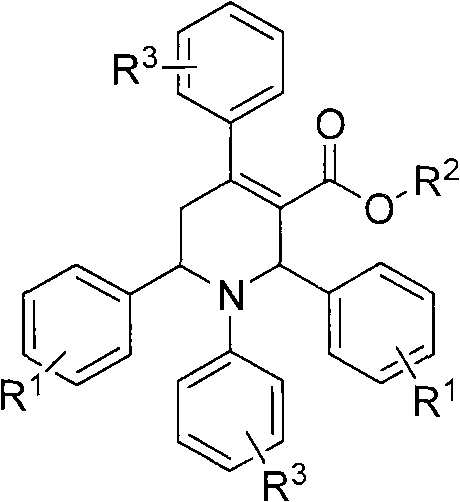
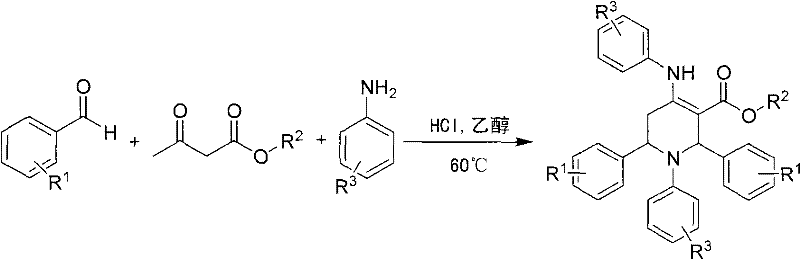
Method for preparing polysubstituted tetrahydropyridine
A technology of tetrahydropyridine and multi-substitution, which is applied in the field of preparation of multi-substituted tetrahydropyridine, can solve the problems of non-conforming to green chemistry, impossibility of practical application, and many side reactions, and achieve economical steps, less pollution, and simple and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
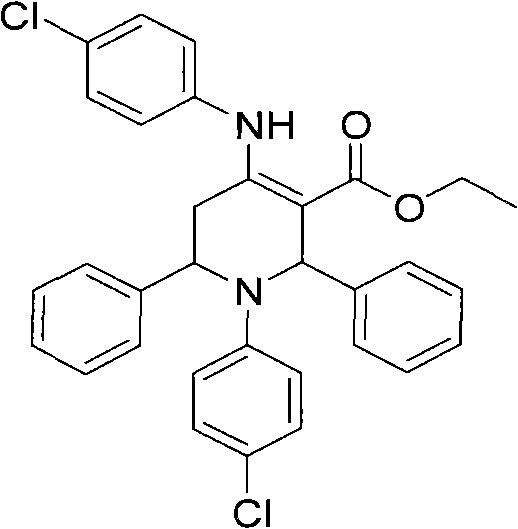
[0021] Example 1, 1-(4-chlorophenyl)-4-(4-chloroanilino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-ethyl carbonate preparation
[0022] In the reaction flask (50mL round bottom flask) that has added ethanol (2mL), add benzaldehyde (0.212g, 2mmol), ethyl acetoacetate (0.260g, 2mmol), 4-chloroaniline (0.256g, 2mmol), Add 0.02mL of concentrated hydrochloric acid with a concentration of 12mol / L dropwise, and stir at 60°C for 23 hours at a controlled temperature; after the reaction, add ethyl acetate (15mL) and water (5mL) into the reaction flask, stir for 10 minutes, and then let it stand , liquid separation, the organic phase was separated, and the aqueous phase was extracted 3 times with ethyl acetate (10mL), and all the organic phases were combined, and the solvent was evaporated and recrystallized with ethanol-DMF (3 times), and finally the pure product 1 was obtained. -(4-Chlorophenyl)-4-(4-chloroanilino)-2,6-diphenyl-1,2,5,6-tetrahydropyridine-3-ethyl carbonate. Yield: 80%...
Embodiment 2
[0033] Example 2, 1-phenyl-4-anilino-2,6-bis(3,4-dimethoxyphenyl)-1,2,5,6-tetrahydropyridine-3-allyl carbonate Preparation of esters
[0034]In the reaction flask (50mL round bottom flask) that has added ethanol (2mL), add 3,4-dimethoxybenzaldehyde (0.332g, 2mmol), allyl acetoacetate (0.284g, 2mmol), aniline ( 0.186g, 2mmol), and then dropwise added 0.02mL of concentrated hydrochloric acid with a concentration of 12mol / L, controlled the temperature and stirred at 60°C for 19 hours; added ethyl acetate (15mL) and water (5mL ), stirred for 10 minutes and allowed to stand, separated the liquid, separated the organic phase, and extracted the aqueous phase with ethyl acetate (10mL) for 3 times, combined all the organic phases, evaporated the solvent and carried out recrystallization with ethanol-DMF (3 time), finally got the pure product 1-phenyl-4-anilino-2,6-bis(3,4-dimethoxyphenyl)-1,2,5,6-tetrahydropyridine-3-carbonic acid Allyl esters. Yield: 87%.
[0035] This product is ...
Embodiment 3
[0045] Example 3, 1-(4-chlorophenyl)-4-(4-chloroanilino)-2,6-bis(2-methoxyphenyl)-1,2,5,6-tetrahydropyridine - Preparation of 3-allyl carbonate
[0046] In the reaction flask (50mL round bottom flask) that has added ethanol (2mL), add 2-methoxybenzaldehyde (0.272g, 2mmol), allyl acetoacetate (0.284g, 2mmol), 4-chloroaniline ( 0.256g, 2mmol), then dropwise added 0.02mL of concentrated hydrochloric acid with a concentration of 12mol / L, controlled the temperature and stirred at 60°C for 19 hours; after the reaction was completed, ethyl acetate (15mL) and water (5mL) were added into the reaction flask, After stirring for 10 minutes, let it stand, separate the liquid, separate the organic phase, extract the aqueous phase with ethyl acetate (10mL) for 3 times, combine all the organic phases, evaporate the solvent and use ethanol-DMF for recrystallization (3 times) , finally get the pure product 1-(4-chlorophenyl)-4-(4-chloroanilino)-2,6-bis(2-methoxyphenyl)-1,2,5,6-tetrahydro Pyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com