Process for manufacturing acrolein or acrylic acid from glycerin
一种丙烯醛、丙烯酸的技术,应用在碳基化合物制备、化学仪器和方法、有机化合物的制备等方向,能够解决没有催化剂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
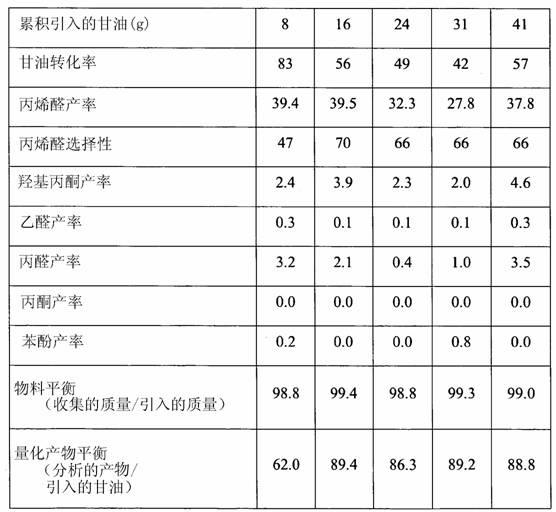
Examples
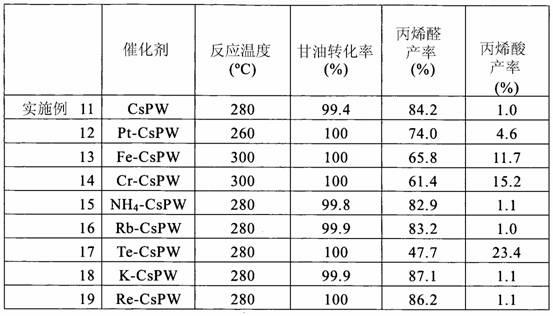
Embodiment 1
[0103] Cesium salt of tungstophosphoric acid (CsPW) was prepared according to JP-A1-4-139149. That is, 50 g tungstophosphoric acid (H 3 [PW 12 o 40 ] nH 2 (2, n=about 30, product of Nippon Inorganic Color & Chemical Co., Ltd) was dissolved in 20 ml of pure water to obtain an aqueous solution of tungstophosphoric acid. In a separate beaker, 7.19 g cesium nitrate (CsNO 3 , Kishida Chemical Co., Ltd) was dissolved in 60 ml of water to obtain an aqueous solution of cesium nitrate. Under stirring, the aqueous solution of cesium nitrate was added dropwise into the aqueous solution of tungstophosphoric acid through the dropping funnel. Each drop produces a white slurry.
[0104] The resulting slurry was processed in a rotary evaporator under vacuum at 60 °C to obtain a white powder. The powder was then dried in an oven at 150°C for 6 hours at ambient pressure. Then, the resulting powder was calcined in air at 250° C. for 3 hours using a muffle furnace to obtain a cesium salt ...
Embodiment 2
[0113] Repeat the method of embodiment 1, except using 5.44 g rubidium nitrate (RbNO 3 ) (Mitsuwa Chemicals Co., Ltd) instead of cesium nitrate (CsNO 3 ) to prepare a rubidium salt catalyst (RbPW) of tungstophosphoric acid having the following composition: H 0.5 Rb 2.5 PW 12 o 40 .
[0114] Reaction and evaluation were performed under the same conditions as in Example 1.
Embodiment 3
[0116] The method of Example 1 was repeated except that 3.22 g of calcium chloride dihydrate (CaCl 2 2H 2 O) (Wako Pure Chemical Industries, Ltd) instead of cesium nitrate (CsNO 3 ) to prepare a calcium salt catalyst (CaPW) of tungstophosphoric acid having the following composition: Ca 1.5 PW 12 o 40 .
[0117] Reaction and evaluation were performed under the same conditions as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com