Quality control method for phlegm eliminating and asthma relieving cough syrup
A quality control method and technology for cough syrup, applied in the quality control field of expectorant, asthma and cough syrup, can solve the problems of no identification item, no reflected drug content determination item, poor qualitative analysis, etc., so as to improve quality control and ensure internal Quality and efficacy, the effect of perfect quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
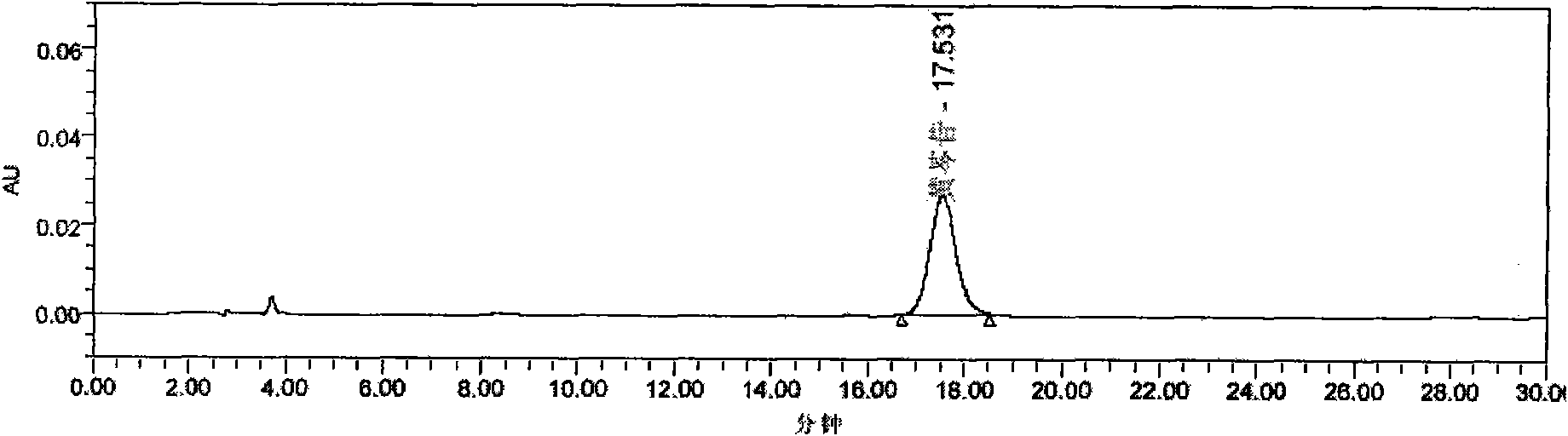
[0039] Preparation of Reference Substance Solution Take an appropriate amount of baicalin reference substance, weigh it accurately, add methanol to make a solution containing 0.06mg per 1ml, and obtain it.
[0040] Preparation of the test solution Precisely draw 1ml of this product and put it in a 10ml volumetric flask, dilute to the mark with 70% ethanol, shake fully, centrifuge, filter, and use it as the test solution.
[0041] Determination method Precisely draw 5 μl each of the reference substance solution and the test solution, inject it into the liquid chromatograph, measure it, and obtain it. This product contains not less than 0.6mg of baicalin per ml.
[0042] Functions and indications: anti-inflammatory cough, expectorant and asthma. For mild and medium chronic bronchitis, cough and asthma. Usage and dosage: take orally, 10ml each time, twice a day. Specifications: 100ml per bottle; Storage: Sealed and kept in a cool place.
Embodiment 2
[0043] Example 2 Drafting of Great Wall Zhikechuan Syrup Quality Standard Description: There is no identification and content determination in the original quality standard of Great Wall Zhikechuan Syrup. The TLC identification of Scutellaria baicalensis and Scutellaria baicalensis and the method of content determination of Scutellaria baicalensis were researched and tested, and the results were increased. TLC identification of red and scutellaria baicalensis; in addition, the content of baicalin in Great Wall Zhikechuan Syrup was determined by HPLC method, and the content of baicalin was determined.
[0044] 2. TLC identification
[0045] 1. Identification of Manshanhong
[0046] 1.1 Materials and reagents
[0047] Ethanol, ether, toluene, ethyl acetate, formic acid: analytically pure
[0048] Manshanhong reference medicinal material: China National Institute for the Control of Pharmaceutical and Biological Products, batch number: 1035-200003 high-efficiency silica gel G th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com