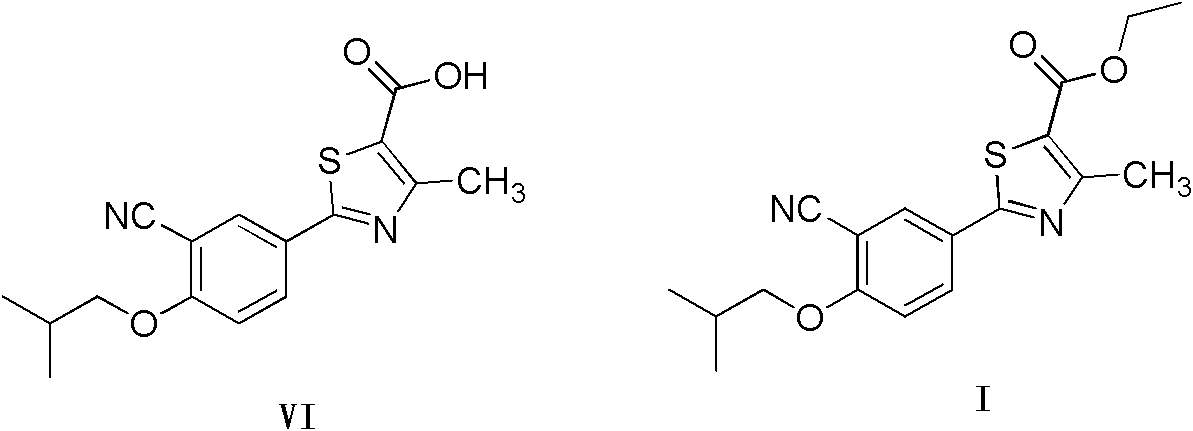
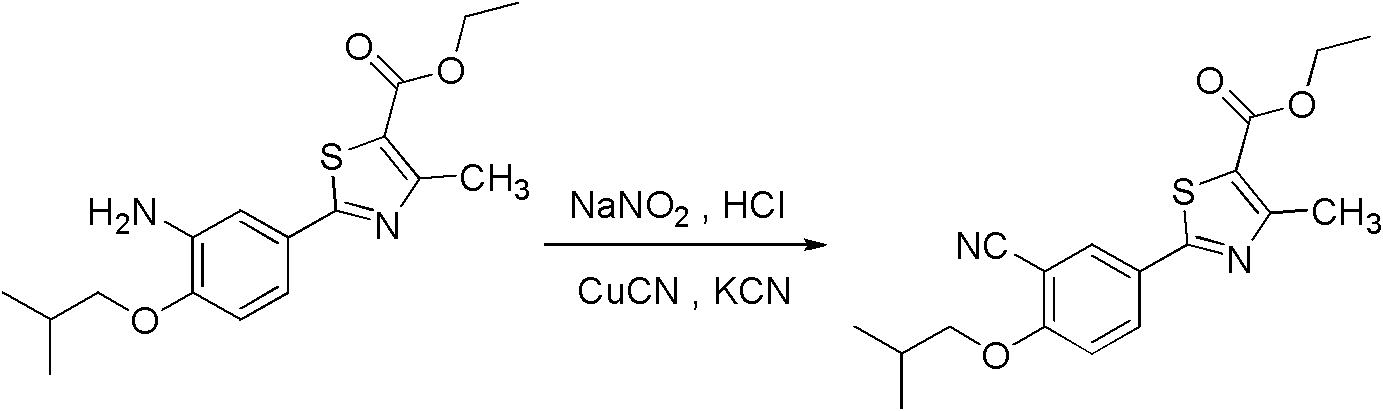
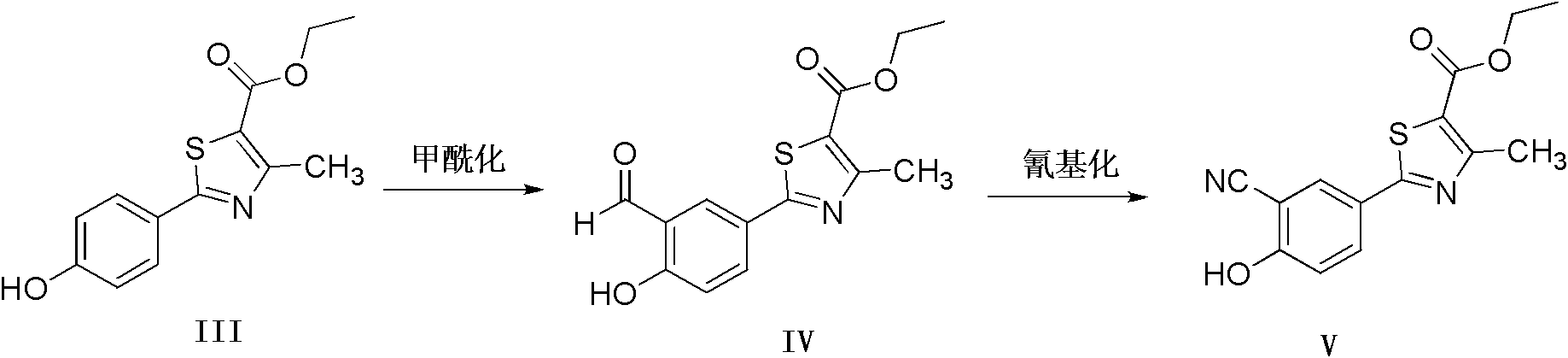
Method for preparing 2-(3-cyano-4-isobutyl methoxyphenyl)-4-methylthiazol-5-ethyl formate
A technology of isobutyloxyphenyl and methylthiazole, which is applied in the field of chemical drug preparation, can solve the problems of restricting industrialization, and achieve the effect of low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Reaction steps (1), (2), (4) are the same as above, and reaction steps (3), (5) are:
[0031] (3) Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate (compound IV)
[0032] In a 1000mL three-neck flask, add 90g of compound III and 420mL of methyl tetrahydrofuran, after stirring, add 39g of anhydrous magnesium chloride, add 20.5g of paraformaldehyde in batches, add dropwise 41.5g of triethylamine, and react at 60°C for 12 hours , TLC showed that the reaction was almost complete, concentrated most of the solvent, added dilute hydrochloric acid to adjust the pH to weak acidity, poured into a large amount of water, precipitated solid, filtered and dried to obtain 86g of yellow solid, yield 86.4%.
[0033] (5) 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-ethyl formate, the preparation of (compound I)
[0034] In a 500mL three-neck flask, add 70g of compound V and 180mL of DMF, stir, add 16.8g of hydroxylamine hydrochloride, and react a...
Embodiment 2
[0036] Reaction steps (1), (2), (4) are the same as above, and reaction steps (3), (5) are:
[0037] (3) Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate (compound IV)
[0038] In a 1000mL three-neck flask, add 90g of compound III and 420mL of methyl tetrahydrofuran, after stirring, add 39g of anhydrous magnesium chloride, add 20.5g of paraformaldehyde in batches, add dropwise 41.5g of triethylamine, and react at 60°C for 12 hours , TLC showed that the reaction was almost complete, concentrated most of the solvent, added dilute hydrochloric acid to adjust the pH to weak acidity, poured into a large amount of water, precipitated solid, filtered and dried to obtain 86g of yellow solid, yield 86.4%.
[0039] (5) 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-ethyl formate, the preparation of (compound I)
[0040]In a 500mL three-neck flask, add 70g of compound V and 180mL of DMF, stir, add 28g of hydroxylamine hydrochloride, and react at 1...
Embodiment 3
[0042] Reaction steps (1), (2), (4) are the same as above, and reaction steps (3), (5) are:
[0043] (3) Preparation of ethyl 2-(3-formaldehyde-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate (compound IV)
[0044] In a 1000mL three-neck flask, add 90g of compound III and 420mL of methyl tetrahydrofuran, after stirring, add 39g of anhydrous magnesium chloride, add 20.5g of paraformaldehyde in batches, add dropwise 41.5g of triethylamine, and react at 60°C for 12 hours , TLC showed that the reaction was almost complete, concentrated most of the solvent, added dilute hydrochloric acid to adjust the pH to weak acidity, poured into a large amount of water, precipitated solid, filtered and dried to obtain 86g of yellow solid, yield 86.4%.
[0045] (5) 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-ethyl formate, the preparation of (compound I)
[0046] In a 500mL three-neck flask, add 70g of compound V and 180mL of DMF, stir, add 22.4g of hydroxylamine hydrochloride, and react a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com