Deoxy-podophyllotoxin type compound and preparation and application thereof
A technology for methyl deoxypodophylloyloxyformyl and compounds, which is applied in the field of deoxypodophyllotoxin compounds, can solve the problems that water solubility has not been fundamentally improved and the stability of compounds is not high, and achieves mild conditions, simple methods, and raw materials. Inexpensive and easy to get effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
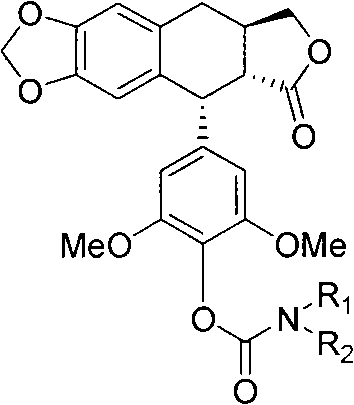
[0033] 4′-Desmethyl deoxypodophyllo (VIII)
[0034] Get 8.0g of 10% Pd / C and 200mL of acetic acid into the autoclave, under H 2 Stir under atmosphere until no more H 2 Until it is absorbed, take 12.0g (19.3mmol) 4′-norepipodophyllophyllum (VI) and add it, and the mixture is kept at 85°C under 2atm H 2 The reaction was stirred under atmosphere for 5 hours, cooled naturally, the catalyst was filtered off, and concentrated to obtain a crude product. The crude product was further recrystallized from methanol to obtain 5.8 g of white solid. Yield 78%, m.p.: 244-245°C, (c 0.5CHCl 3 ). 1 H NMR (400MHz, CDCl 3 )δ6.66(s, 1H), 6.52(s, 1H), 6.35(s, 2H), 5.94(d, J=9.2Hz, 2H), 5.41(s, 1H), 4.60(s, 1H), 4.45-4.42 (m, 1H), 3.93-3.90 (m, 1H), 3.78 (s, 6H), 3.08-3.05 (m, 1H), 2.77-2.72 (m, 3H).
[0035] In the tumor cell proliferation inhibition experiment described later, the sample number of this embodiment is VII.
Embodiment 2
[0037] 4′-Desmethyl deoxypodophylloyloxyformyl p-nitrophenyl ester (IX)
[0038] Dissolve p-nitrophenyl chloroformyl ester (1.3g, 6.35mmol) in 10ml of dry dichloromethane, and add 0.6ml of dry pyridine. Under the protection of nitrogen, 5 ml of dichloromethane solution dissolved with deoxypodophyllophyllum (7.2 g, 1.87 mmol) was added. The mixture was reacted at room temperature for 1 h. After the reaction, 10 ml of water was added to the above reaction solution, and extracted three times with dichloromethane. The organic layers were combined, washed with water and saturated brine successively, the organic phase was dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 8.9 g of compound IV by column chromatography, yield: 92%. 1 H NMR (400MHz, CDCl 3 )δ8.30(d, J=8.8Hz, 2H, p-Ph), 7.50(d, J=9.2Hz, 2H, p-Ph), 6.68(s, 1H, H-5), 6.52(s, 1H, H-8), 6.43(s, 1H, H-2', 6'), 5.96(s, 2H, OCH 2 O), 4.66(d, J=4.2Hz, 1H, 1-H), 4.49-4.45(m, 1H, H-11a), 3.96-3.9...
Embodiment 3
[0040] 4′-Desmethyl-deoxypodophyllooxyformyl 4-N-4-methylphenylpiperazine amide
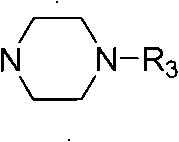
[0041] 110 mg (0.2 mmol) of compound IX was dissolved in 5 ml of dry dichloromethane, and N-methylpiperazine (22 mg, 0.22 mmol) and triethylamine (25 mg, 0.22 mmol) were added sequentially at room temperature under nitrogen protection. Stirring was continued until the reaction was completed, and a white solid compound Ia was obtained by direct column chromatography. The product detection data are as follows:
[0042] Yield: 72%; m.p.: 232-234°C; (c 0.3, CHCl 3 ); IR (cm -1 )3397, 2923, 1771, 1722, 1599, 1483, 1458, 1421, 1228, 1038, 999; 1 H NMR (400MHz, CDCl 3 )δ7.26(s, 1H), 6.66(s, 1H), 6.52(s, 1H), 6.37(s, 2H), 5.94(d, J=6.8Hz, 2H), 4.71(brs, 1H), 4.63(t, J=6.4Hz, 1H), 4.47-4.42(m, 1H), 3.93-3.88(m, 1H), 3.71(brs, 8H), 3.57(br, 2H), 3.08-3.04(m, 1H), 2.80-273(m, 3H), 2.44(t, J=4.4Hz, 4H), 2.33(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ174.8, 153.3, 151.8(2C), 147.0, 146.7, 138.4, 138.7, 130...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com