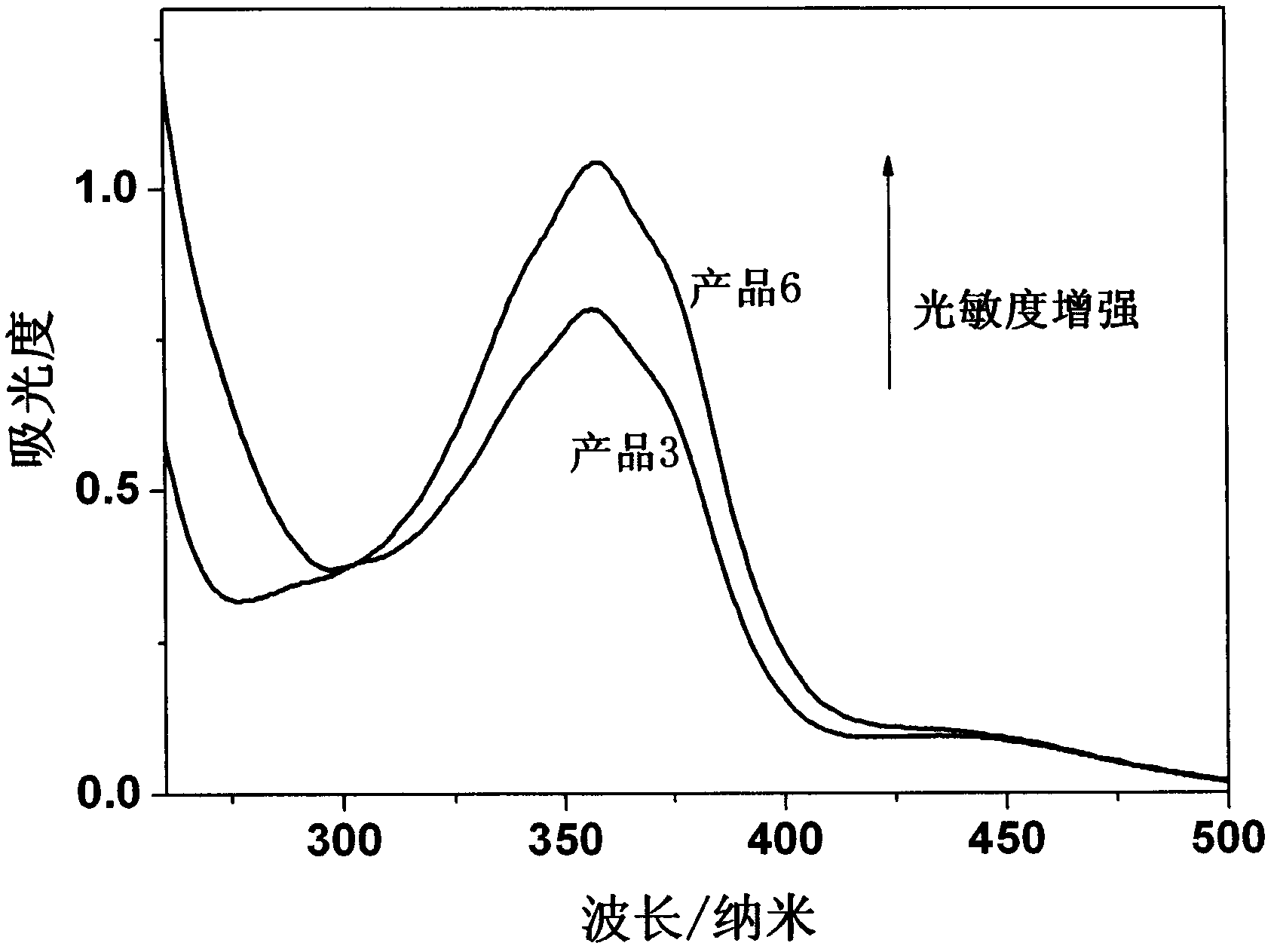
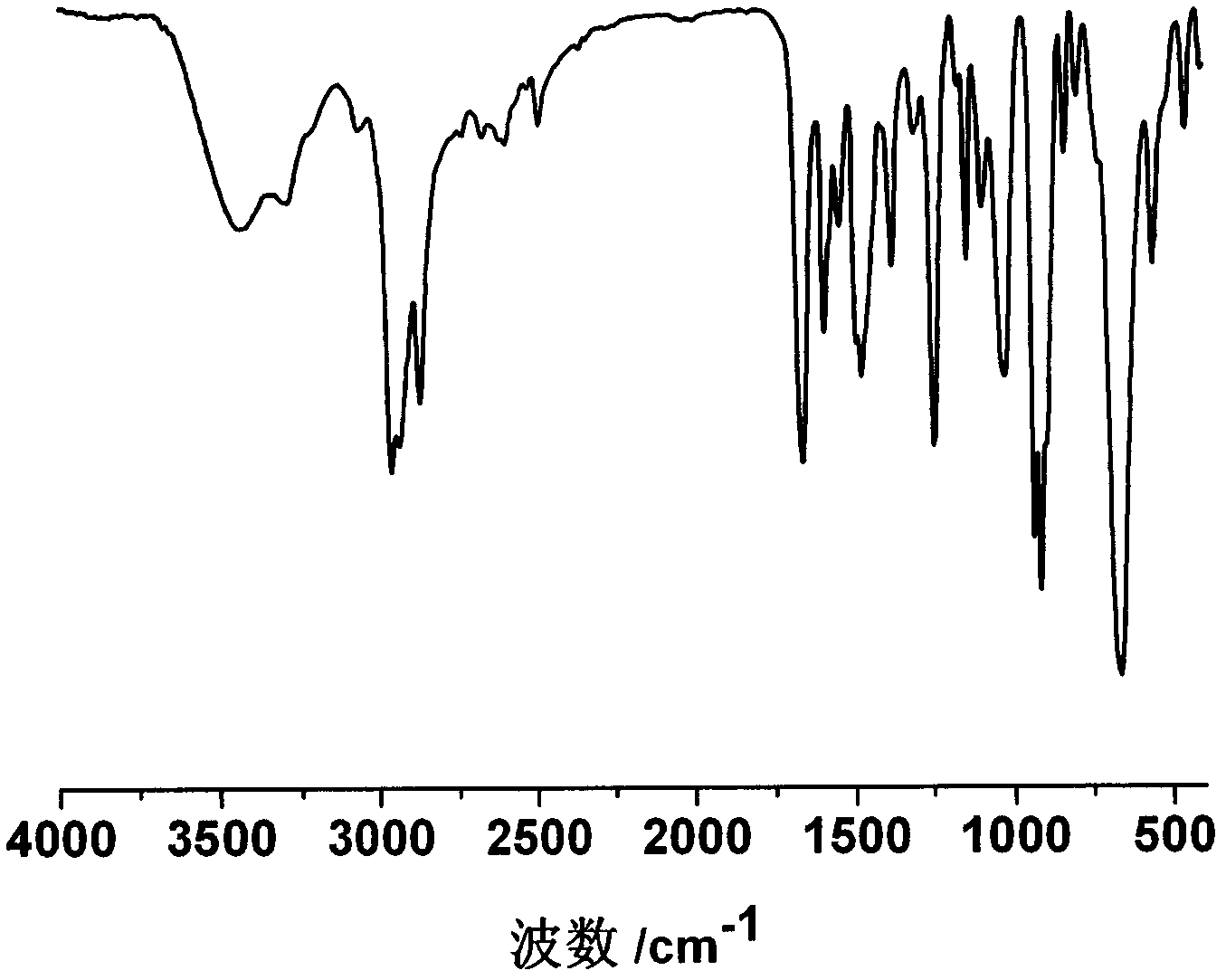
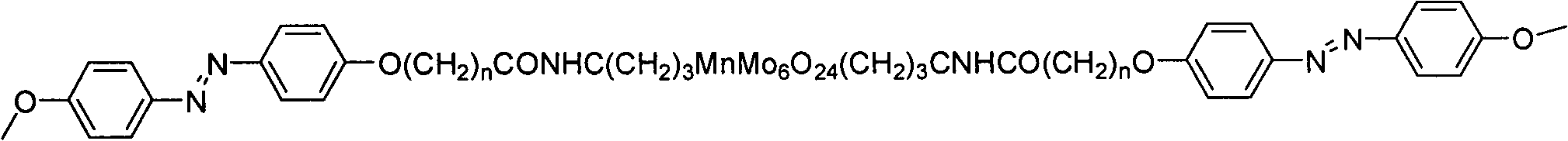Photosensitive material with Anderson type polyacid and preparation method thereof
A photosensitive material and photosensitive technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of few photochromic work reports, and achieve significant changes in absorbance, high yield, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Add 7.5g of 6-bromohexanoic acid into 100ml of methanol, put it in an ice bath, and then add 7.5ml of SOCl dropwise 2 Add dropwise, turn to room temperature, stop reaction after stirring for 8.5 hours, rotary evaporation; Dissolve residue in 100ml ethyl acetate, and use 20ml water successively, 20ml saturated sodium bicarbonate solution, 20ml saturated sodium chloride solution extraction, Take the organic phase and wash it with anhydrous MgSO 4 Drying, filtering, and rotary evaporation gave yellow oily product 1;
[0024] 2. Disperse 4.92g of p-methoxyaniline in 100ml of water, heat at 65°C, then add 10ml of 2M hydrochloric acid, transfer to an ice bath, add 35ml of NaNO with a concentration of 130g / L 2 solution, stirred for 30 minutes, and the concentration of 90g / L phenol solution was dropped into the above solution to obtain a brown solution, adjusted to pH 9, continued to stir for 1 hour, and filtered with suction, and the obtained solid was dried in a vacuum ov...
Embodiment 2
[0031] 1. Add 10.2g of 11-bromoundecanoic acid into 120ml of methanol, put it in an ice bath, and then add 10ml of SOCl dropwise 2 After the dropwise addition, turn to room temperature, stop the reaction after stirring for 6 hours, and rotate to evaporate; the residue is dissolved in 90ml ethyl acetate, and is successively extracted with 30ml water, 25ml saturated sodium bicarbonate solution, and 25ml saturated sodium chloride solution. Take the organic phase and wash it with anhydrous MgSO 4 Drying, filtering, and rotary evaporation gave yellow oily product 7;
[0032] 2. Disperse 4.9g of p-methoxyaniline in 100ml of water, heat at 65°C, then add 10.0ml of 2M hydrochloric acid, transfer to an ice bath, add 20ml of NaNO with a concentration of 95g / L 2 The solution was stirred for 30 minutes, the phenol solution with a concentration of 72 g / L was dropped into the above solution, the pH value was adjusted to 9, the stirring was continued for 1 hour, and the solid was dried in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com