Phosphorus-containing crown macromolecular hybrid nano material as well as preparation and application thereof
A technology of nanomaterials and macromolecules, which is applied in the field of phosphorus-containing macromolecular hybrid nanomaterials and their preparation and application, can solve the problems of undiscovered tumor gene silencing and chemotherapy combined therapy, and achieve easy operation, separation, and final product Uniform molecular weight and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
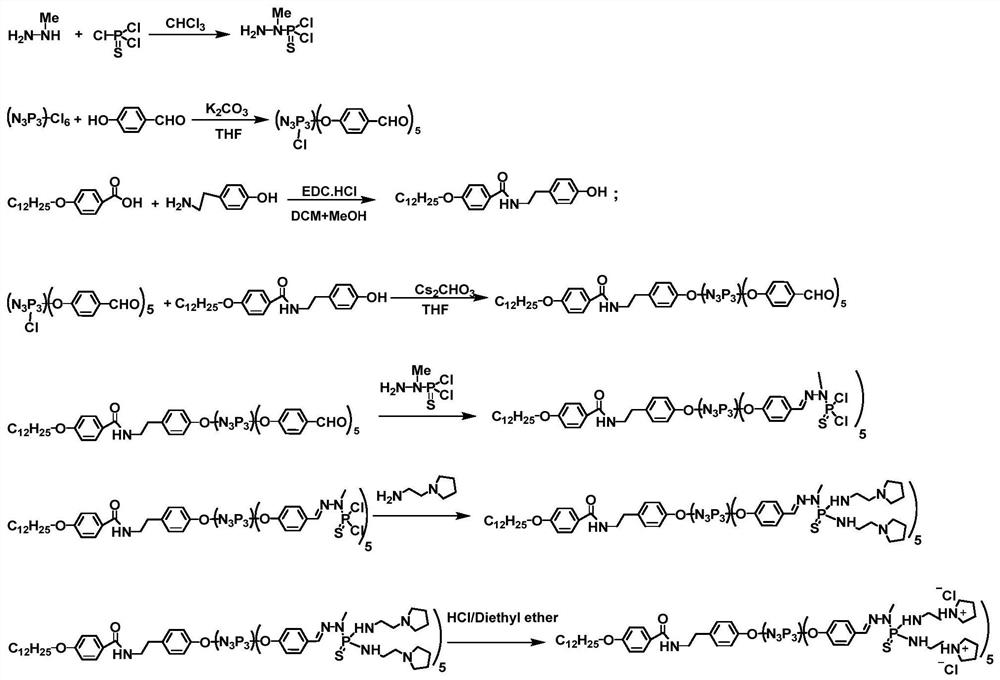
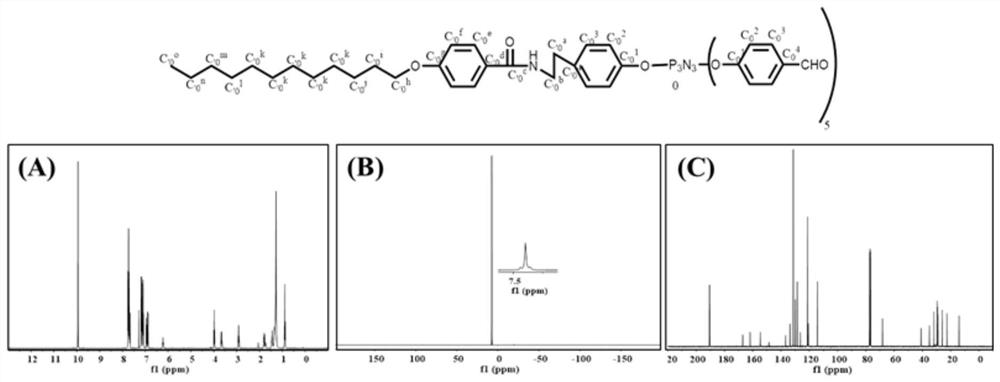
[0061] (1) Dissolve hexachlorocyclotriphosphazene (0.4mmol) in 10mL of anhydrous tetrahydrofuran, add anhydrous cesium carbonate (4.8mmol) for activation; then add 10mL of tetrahydrofuran dissolved in p-hydroxybenzaldehyde (2.0mmol) dropwise Solution, reacted at room temperature for 12 hours, thin layer chromatography (TLC) detection reaction process, filtered to remove precipitate, then purified by column chromatography (ethyl acetate and n-hexane, v:v=1:1.5), and finally vacuum dried to obtain AB 5 (MW=775).
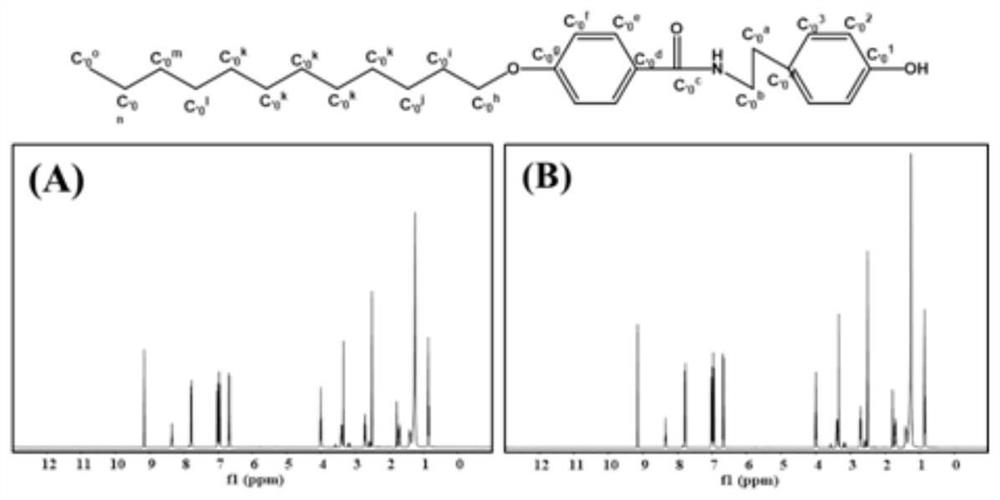
[0062] (2) Dissolve 4-dodecyloxybenzoic acid (3.64mmol) in 10mL of anhydrous dichloromethane, add EDC*HCl (3.64mmol) for activation; then add 10mL of tyramine (3.64mmol) dropwise methanol solution, reacted at room temperature for 12 hours, thin layer chromatography (TLC) to detect the reaction process, purified by column chromatography (methanol and dichloromethane, v:v=1:19), and finally vacuum-dried to obtain amide (C12H25, MW=425).
[0063] (3) Dissolve C12H25 (0...
Embodiment 2
[0090] Prepare solutions of 1-C12G1 at concentrations ranging from 0.001 mg / mL to 2 mg / mL. Add the prepared solution to the solution containing the fluorescent probe pyrene (10 μL, 4.0×10 -4 M) in the flask, the final concentration of pyrene is 6.0 × 10 -7 M. Sonicate for 30 minutes and store overnight at room temperature to determine micelle formation. A fluorescence spectrophotometer measures the fluorescence spectrum with an excitation wavelength of 335 nm, and an excitation and emission bandwidth of 5 nm. Analyze the lg value of 1-C12G1 concentration and the fluorescence intensity ratio of I373 / I393 as the abscissa and ordinate (as attached Image 6 shown). The results showed that the fluorescence intensity ratio of I373 / I393 decreased significantly at 151 μM with the increase of 1-C12G1 concentration, which indicated that the material 1-C12G1 could form micelles, and the critical micelle concentration was 151 μM.
Embodiment 3
[0092] Doxorubicin (DOX) was dissolved in methanol, and then a certain amount of DOX methanol solution was added to the 1-C12G1 in aqueous solution, stirred overnight at room temperature. Then transfer the mixed solution into a centrifuge tube, centrifuge twice at 7000 rpm for 20 minutes, take out the supernatant after each centrifugation, resuspend the precipitate with an appropriate amount of ultrapure water, and then perform the next centrifugation. Dissolve the precipitate in 1 mL of methanol, measure its UV absorbance at 481 nm, and calculate the encapsulation efficiency and loading efficiency of DOX by comparing with the standard curve of pure DOX (Table 2). The results showed that as the molar ratio of 1-C12G1 to DOX increased, the encapsulation efficiency and uploading rate of DOX gradually increased, and when it reached 1:30, the uploading rate reached the maximum value, so 1:30 was chosen as the best of both Mixing ratio for subsequent experiments.
[0093] 1-C12G1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com