Preparation and application of iron oxide/polyaniline composite anode
A composite anode, iron oxide technology, used in battery electrodes, biochemical fuel cells, electrical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
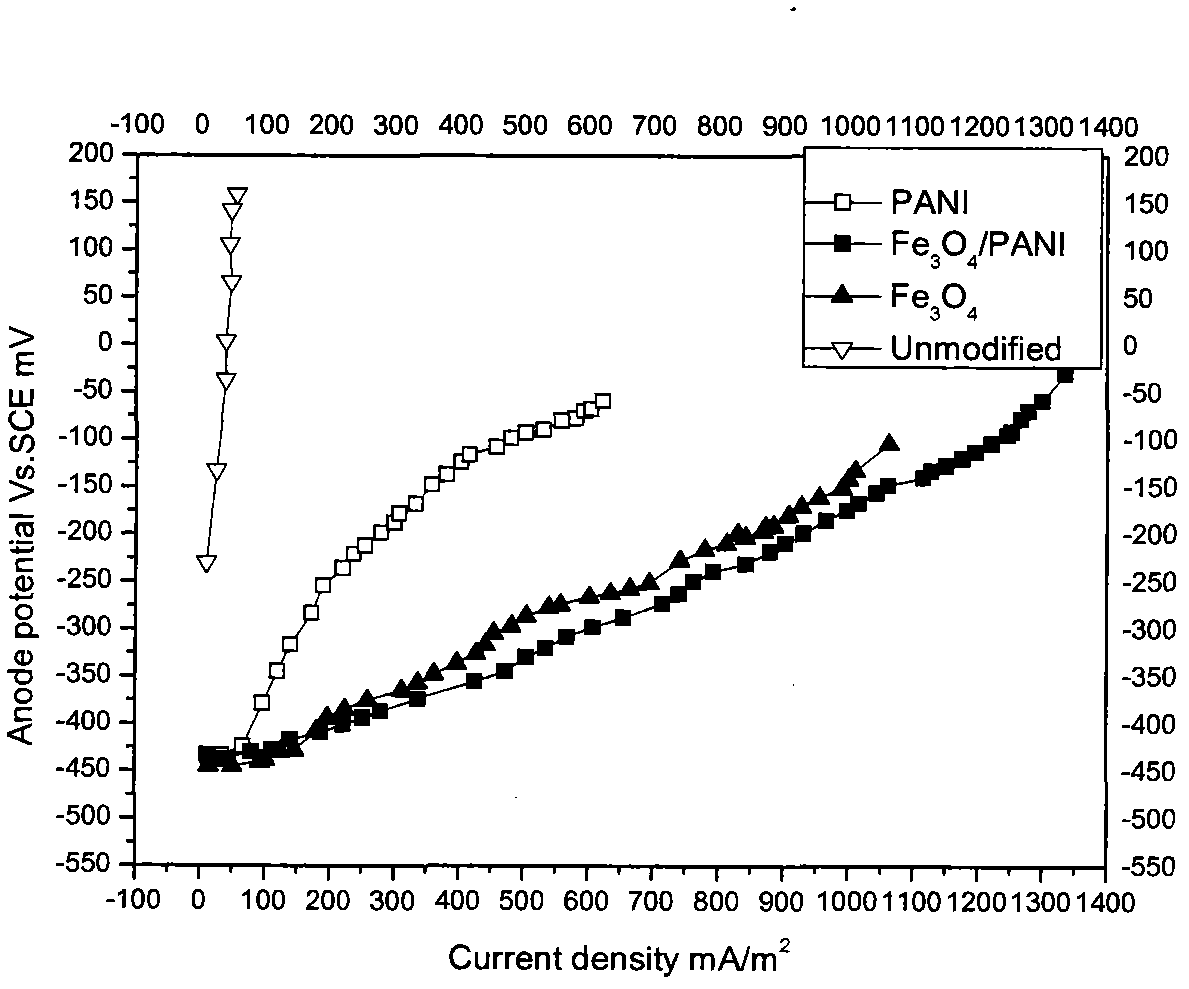
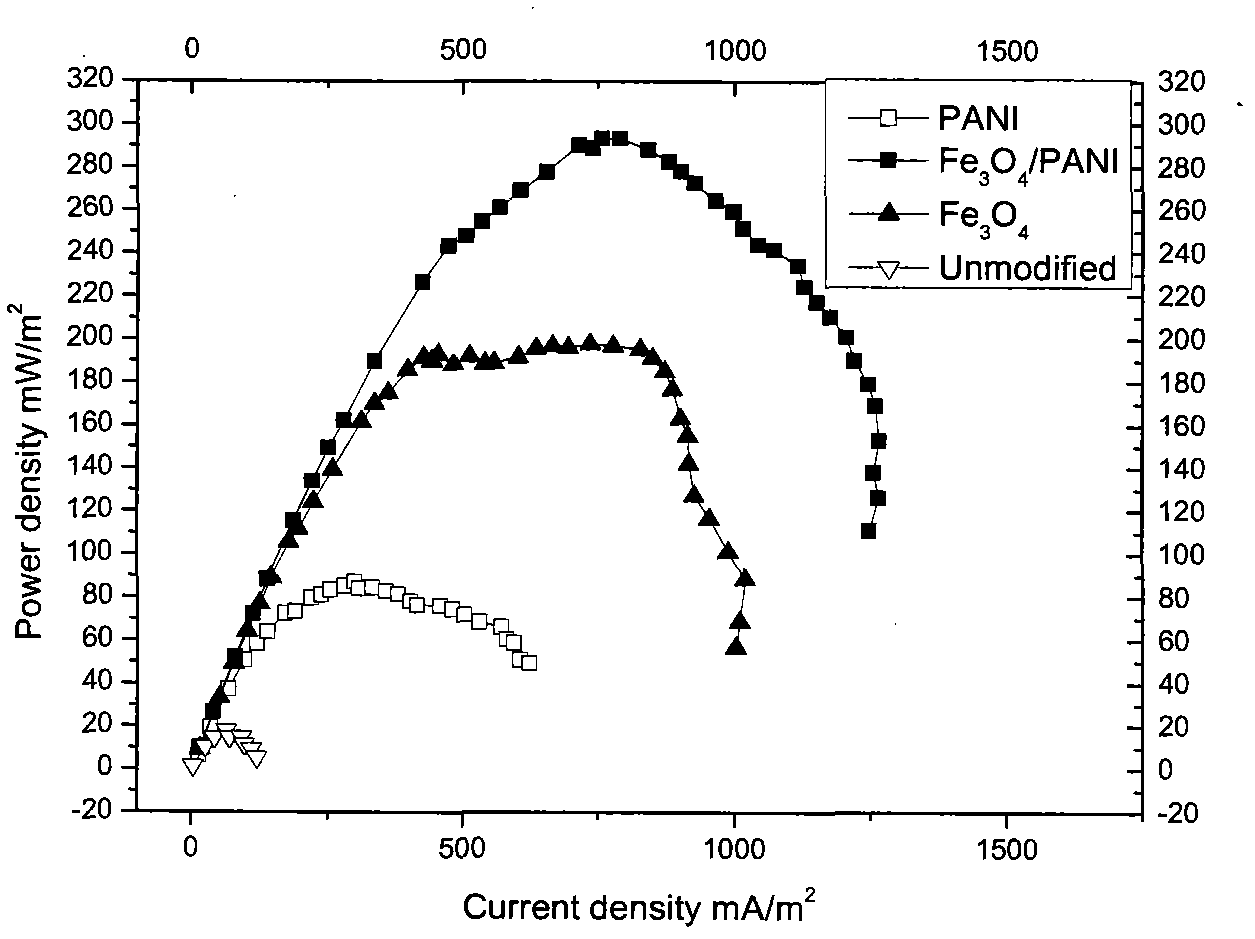
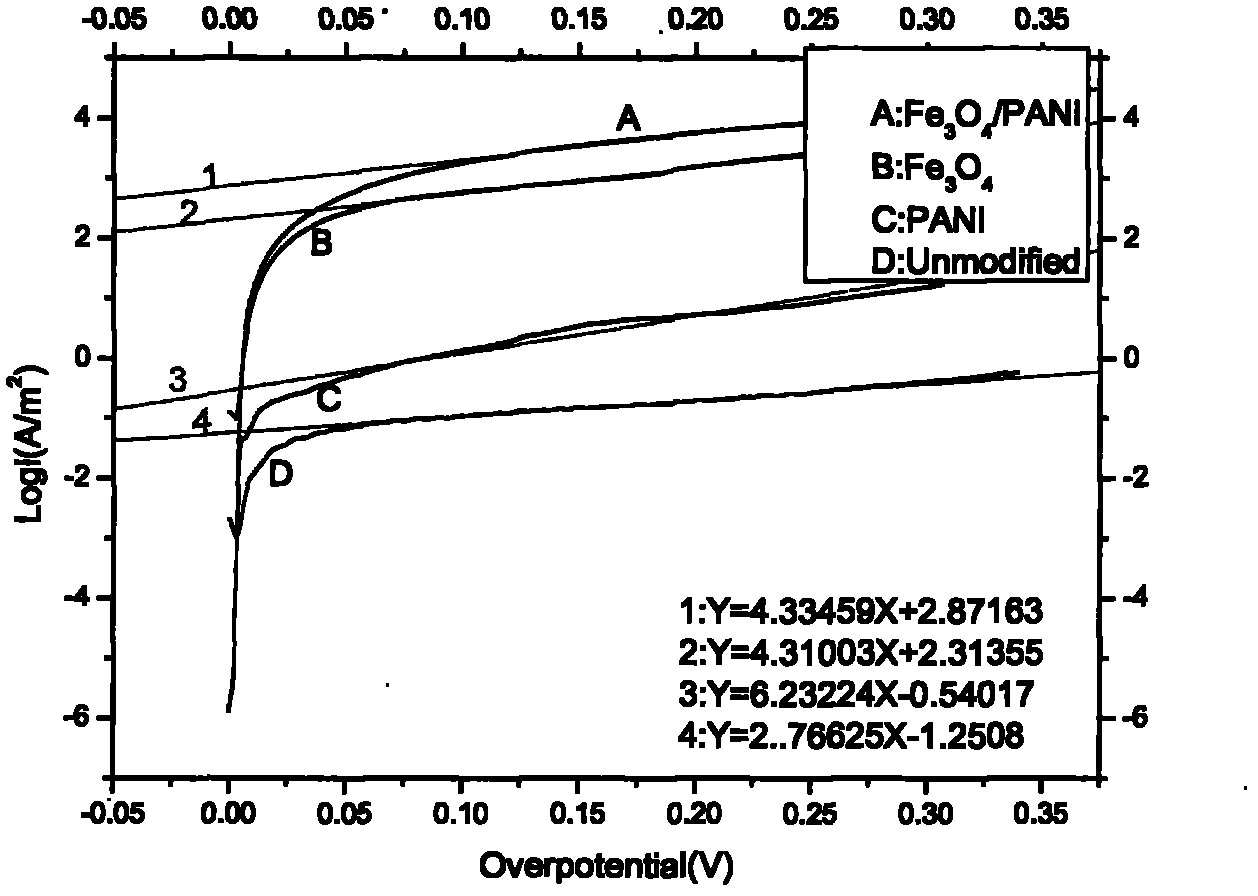
Image
Examples
Embodiment 1
[0042] Example 1: This example is used to illustrate the preparation method of iron oxide / polyaniline composite anode.
[0043] Take 2.140g FeCl 2 4H 2 O and 4.967g FeCl 3 ·6H 2 0. Add 60mL of distilled water into a 250mL three-necked round-bottomed flask, stir rapidly until the iron salt is completely dissolved, then raise the temperature to 40°C, add 20mL of concentrated ammonia water, and stir vigorously for 30min, the system turns from transparent yellow-green to black. Then the temperature was raised to 80°C, maintained at this temperature for 30 minutes, and then lowered to room temperature, and the product was separated and collected by magnetic field. Stir and disperse the product with deionized water, and then separate it with a magnetic field. The above process is repeated several times until there is no free Cl - . Add 50ml of distilled water and 1.25g of camphorsulfonic acid into a 250mL three-necked round-bottomed flask, mix and stir for 15min, add 0.5ml of a...
Embodiment 2
[0044] Example 2: This example is used to illustrate the preparation method of iron oxide / polyaniline composite anode.
[0045] Take 1.070g FeCl 2 4H 2 O and 2.483g FeCl 3 ·6H 2 0. Add 60mL of distilled water into a 250mL three-necked round-bottomed flask, stir rapidly until the iron salt is completely dissolved, then raise the temperature to 40°C, add 20mL of concentrated ammonia water, and stir vigorously for 30min, the system turns from transparent yellow-green to black. Then the temperature was raised to 80°C, maintained at this temperature for 30 minutes, and then lowered to room temperature, and the product was separated and collected by magnetic field. Stir and disperse the product with deionized water, and then separate it with a magnetic field. The above process is repeated several times until there is no free Cl - . Add 50ml of distilled water and 1.25g of camphorsulfonic acid into a 250mL three-necked round-bottomed flask, mix and stir for 15min, add 0.5ml of a...
Embodiment 3
[0046] Example 3: This example is used to illustrate the preparation method of iron oxide / polyaniline composite anode.
[0047] Take 4.280g FeCl 2 4H 2 O and 9.934 g FeCl 3 ·6H 2 0. Add 60mL of distilled water into a 250mL three-necked round-bottomed flask, stir rapidly until the iron salt is completely dissolved, then raise the temperature to 40°C, add 20mL of concentrated ammonia water, and stir vigorously for 30min, the system turns from transparent yellow-green to black. Then the temperature was raised to 80°C, maintained at this temperature for 30 minutes, and then lowered to room temperature, and the product was separated and collected by magnetic field. Stir and disperse the product with deionized water, and then separate it with a magnetic field. The above process is repeated several times until there is no free Cl - . Add 50ml of distilled water and 1.25g of camphorsulfonic acid into a 250mL three-necked round-bottomed flask, mix and stir for 15min, add 0.5ml of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com