Niacin salvia miltiorrhiza phenolic ester derivative, preparation method and application thereof
A technology of nicotinic acid salvia and phenolic ester, applied in the directions of drug combination, steroids, cardiovascular system diseases, etc., can solve the problems of unstable tanshinones, low bioavailability, limited application, etc., to improve bioavailability and its stability, inhibition of flushing and hepatotoxicity, and the effect of convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
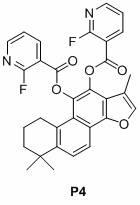
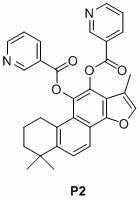
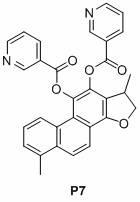
[0037] Embodiment 1, nicotinic acid salvianol ester compound P1 Synthesis.
[0038] Cryptotanshinone (0.30 g) was dissolved in ethyl acetate (50 ml), and palladium on carbon (5%, 15.0 mg) was added. The air in the reaction system was pumped out with a water pump, and hydrogen gas was introduced, and this was repeated three times, so that the air in the reaction system was completely discharged. Regulate the hydrogen pressure in the system to be 1 atmosphere. After stirring for 10 minutes, a solution of nicotinic anhydride (0.50 g) and DMAP (0.30 g) in ethyl acetate was added to the reaction mixture. Stirring was continued for 2 hours and filtered. The filtrate was washed with water (17 ml X 3), dried over anhydrous magnesium sulfate, and concentrated. The residue was subjected to silica gel column chromatography to obtain the target compound P1. The eluent was a mixture of ethyl acetate and petroleum ether (1:1). compound P1 The structural formula and characterization a...
Embodiment 2
[0041] Embodiment 2, nicotinic acid salvianol ester compound P1 Synthesis.
[0042] The method is the same as in Example 1, except that Raney nickel (20.0 mg) is used instead of palladium carbon, and the target product is also obtained P1 .
Embodiment 3
[0043] Embodiment 3, nicotinic acid salvianol ester compound P1 Synthesis
[0044] The method is the same as in Example 1, except that platinum dioxide (6 mg) is used instead of palladium carbon, and after catalytic hydrogenation for 5 minutes, nicotinic anhydride solution is added for reaction, and the target product is also obtained P1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com