Aplotaxis carminative sustained-release preparation and preparation method thereof
A slow-release preparation and woody fragrance technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, block delivery, etc., to achieve the effect of rich variety, unique technology and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
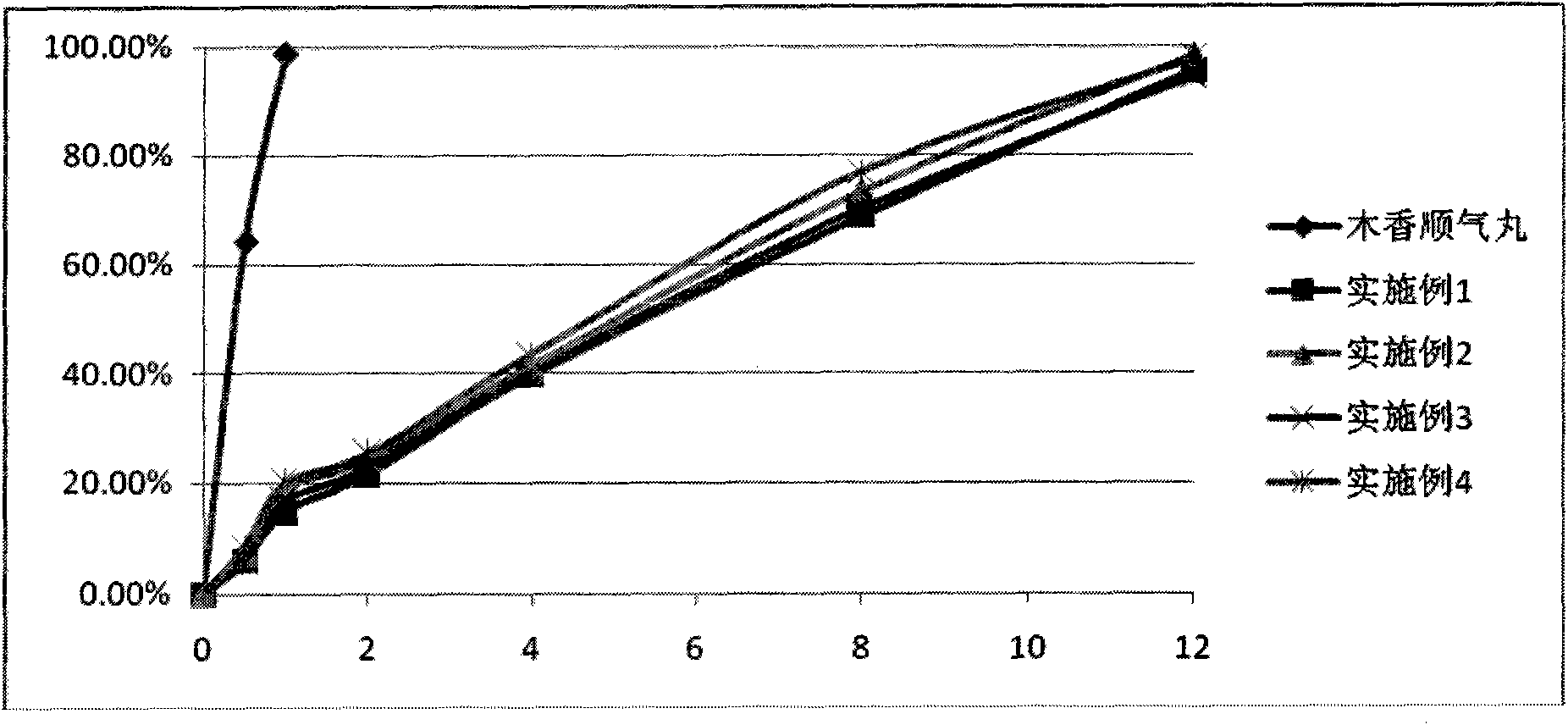
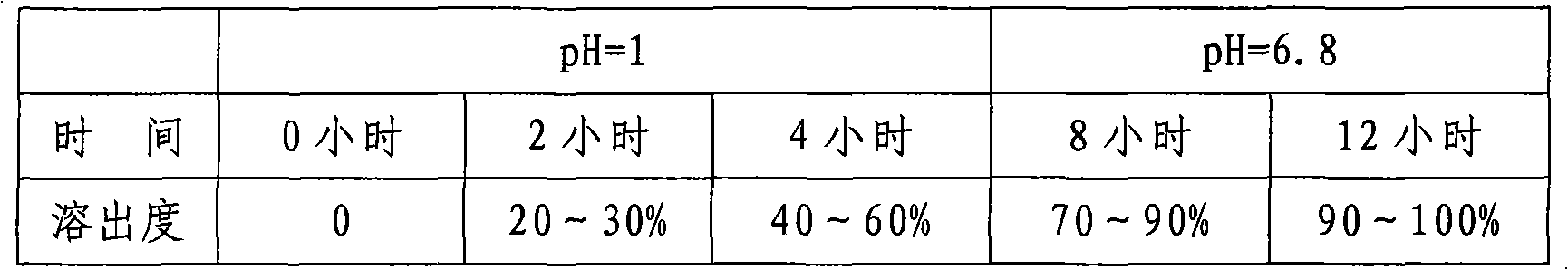
Image
Examples
Embodiment 1
[0021] Preparation of Muxiang Shunqi Sustained-release Tablets
[0022] Prescription: 20 parts of Muxiang Shunqi prescription drug extract, 20 parts of hypromellose (HPMC), 100 parts of polyvinylpyrrolidone (Kollidon), 10 parts of cross-linked polyvinylpyrrolidone, 2 parts of micronized silica gel, stearic acid Magnesium 2 parts.
[0023] Process: take 20 parts of the extract, 100 parts of Kollidon, and 20 parts of HPMC and pass through a 100-mesh sieve, and set aside; prepare an intermediate by hot-melt extrusion, test the content of the intermediate, and set aside; crush the intermediate, add 10 parts of cross-linked polyvinylpyrrolidone , 2 parts of micropowder silica gel, 2 parts of magnesium stearate, mix evenly, and press into tablets to obtain.
Embodiment 2
[0025] Preparation of Muxiang Shunqi Sustained-release Tablets
[0026] Prescription: 20 parts of Muxiang Shunqi prescription drug extract, 30 parts of hypromellose (HPMC), 80 parts of polyvinylpyrrolidone (Kollidon), 10 parts of cross-linked polyvinylpyrrolidone, 2 parts of micronized silica gel, stearic acid Magnesium 2 parts.
[0027] Process: Take 20 parts of the extract, 80 parts of Kollidon, and 30 parts of HPMC and pass through a 100-mesh sieve, and set aside; prepare an intermediate by hot-melt extrusion, test the content of the intermediate, set aside; crush the intermediate, add 10 parts of cross-linked polyvinylpyrrolidone , 2 parts of micropowder silica gel, 2 parts of magnesium stearate, mix evenly, and press into tablets to obtain.
Embodiment 3
[0029] Preparation of Muxiang Shunqi Sustained-release Capsules
[0030] Prescription: 20 parts of Muxiang Shunqi prescription drug extract, 80 parts of Kollidon, 30 parts of HPMC, 10 parts of cross-linked polyvinylpyrrolidone, 2 parts of micronized silica gel, and 2 parts of magnesium stearate.
[0031] Process: Take 20 parts of the extract, 80 parts of Kollidon, 30 parts of HPMC, and 10 parts of cross-linked polyvinylpyrrolidone, pass through a 100-mesh sieve, and set aside; prepare an intermediate by hot-melt extrusion, detect the content of the intermediate, and set aside; crush the intermediate, Add 2 parts of micropowdered silica gel and 2 parts of magnesium stearate, mix evenly, pack into capsules, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com