Preparation method and quality control method of arbidol hydrochloride tablet
A technology of Arbidol hydrochloride tablet and quality control method, which is applied in the field of preparation of Arbidol hydrochloride tablet, and can solve the problems of easy moisture absorption and deterioration, high production cost, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
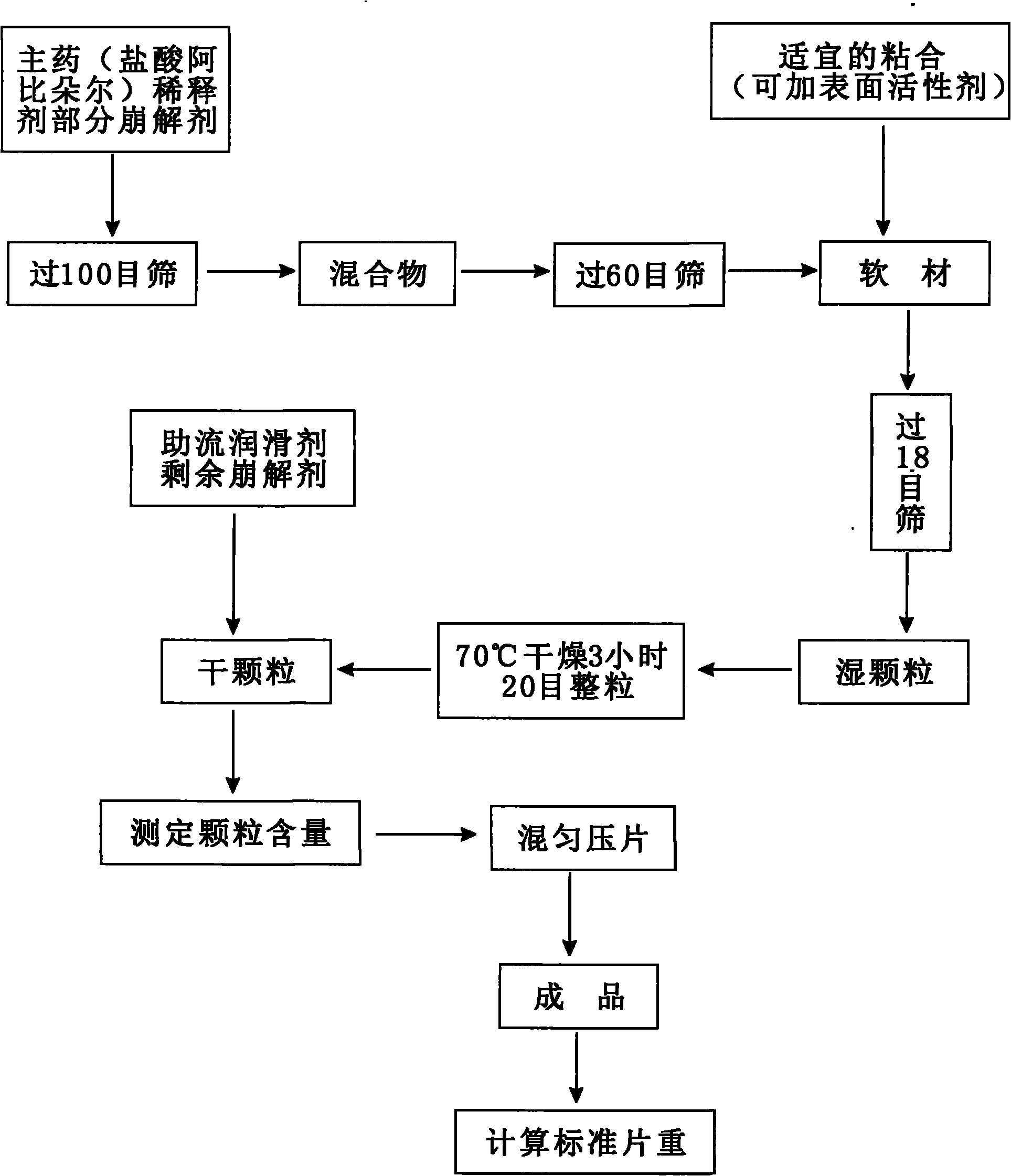
[0029] Embodiment one: figure 1 Its weight ratio (1000 preparation units) is shown in the process flow chart of the preparation method of the present invention.
[0030] Arbidol Hydrochloride 100g
[0031] Microcrystalline Cellulose 20g
[0032] Lactose 38g
[0033] Starch 25g
[0034] Low-substituted hydroxypropyl cellulose 10g
[0037] 6% Adhesive starch slurry Appropriate amount
[0038] Its preparation method:
[0039] 1. Pretreatment: Arbidol hydrochloride, microcrystalline cellulose, lactose, starch, L-hydroxypropyl cellulose, talcum powder, and magnesium stearate are crushed as necessary and passed through a 100-mesh sieve.
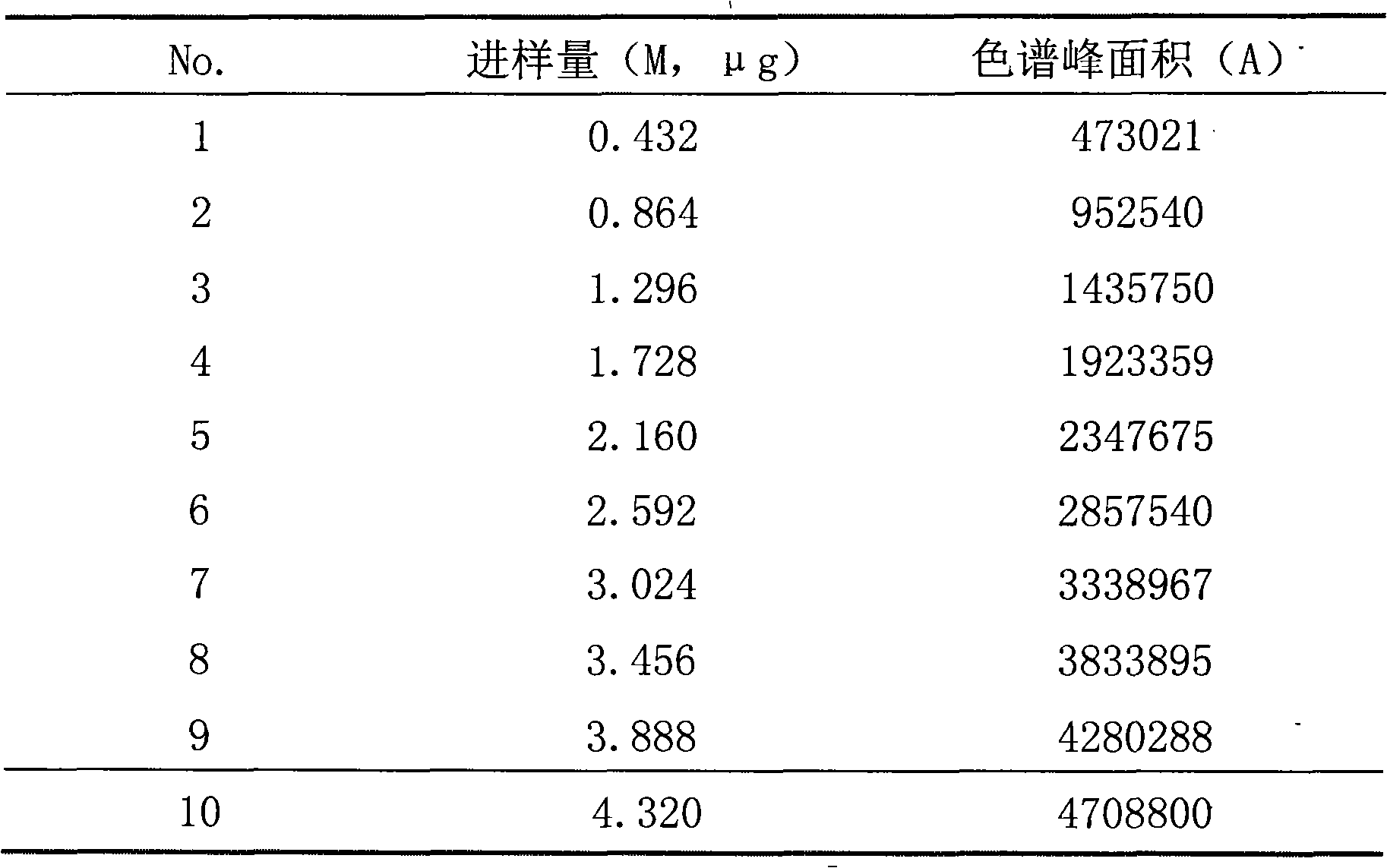
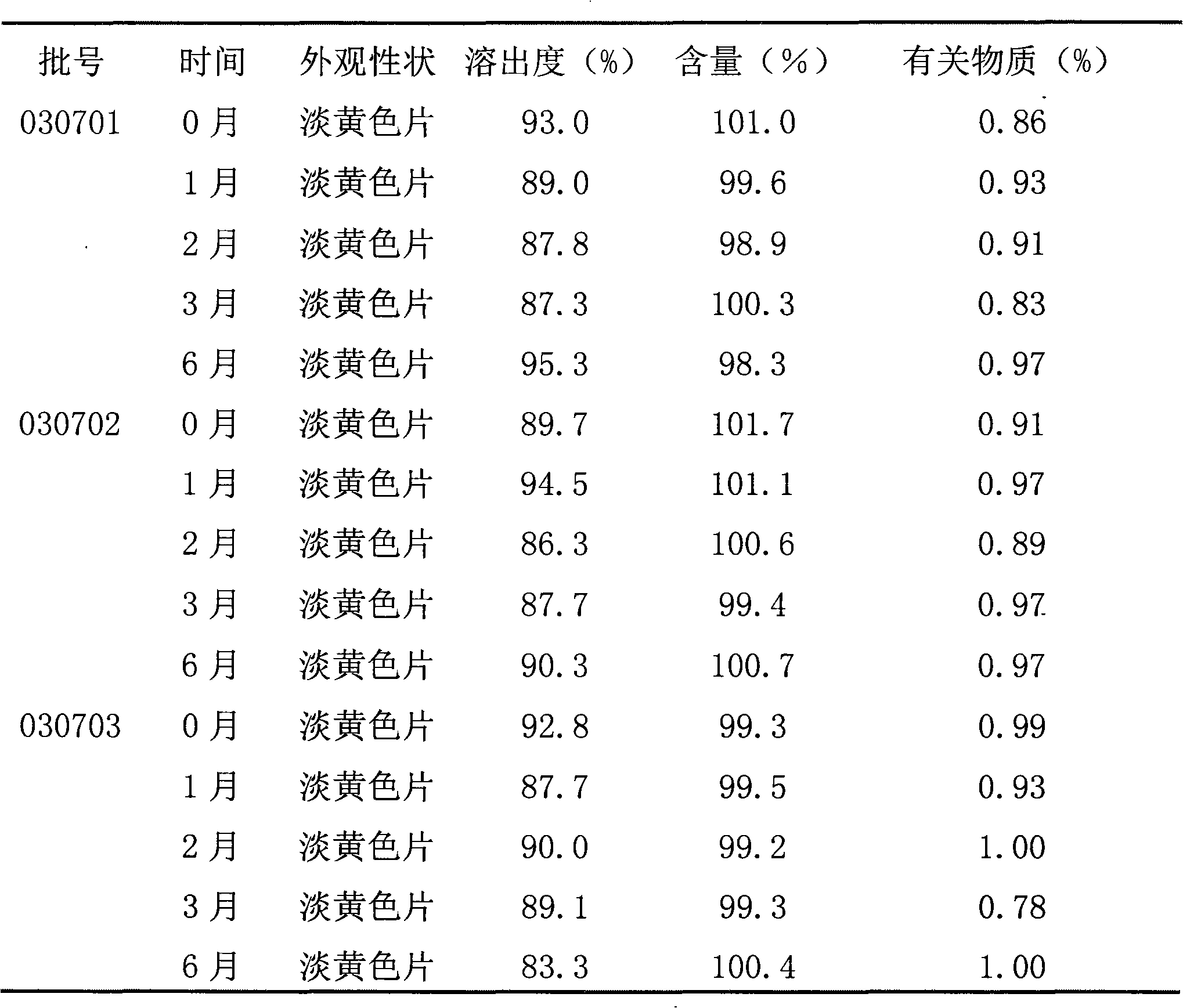
[0040] 2. Preparation of granules: Weigh 5 g of raw materials, microcrystalline cellulose, lactose, starch, and low-substituted hydroxypropyl cellulose according to the prescription, mix evenly, pass through a 60-mesh sieve 10 times, and add an appropriate amount of 6% starch slurry to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com