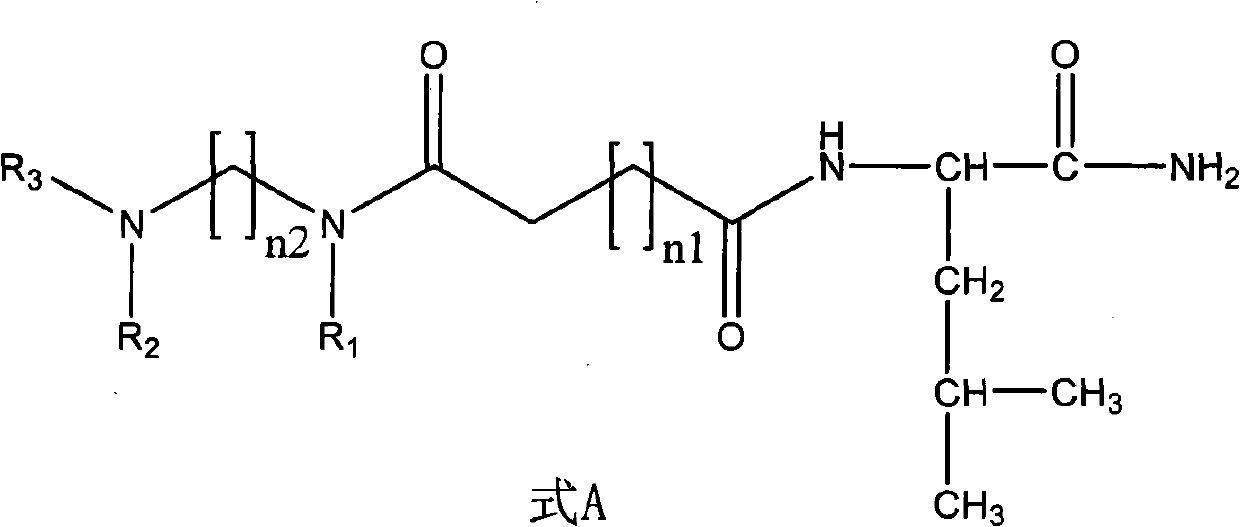
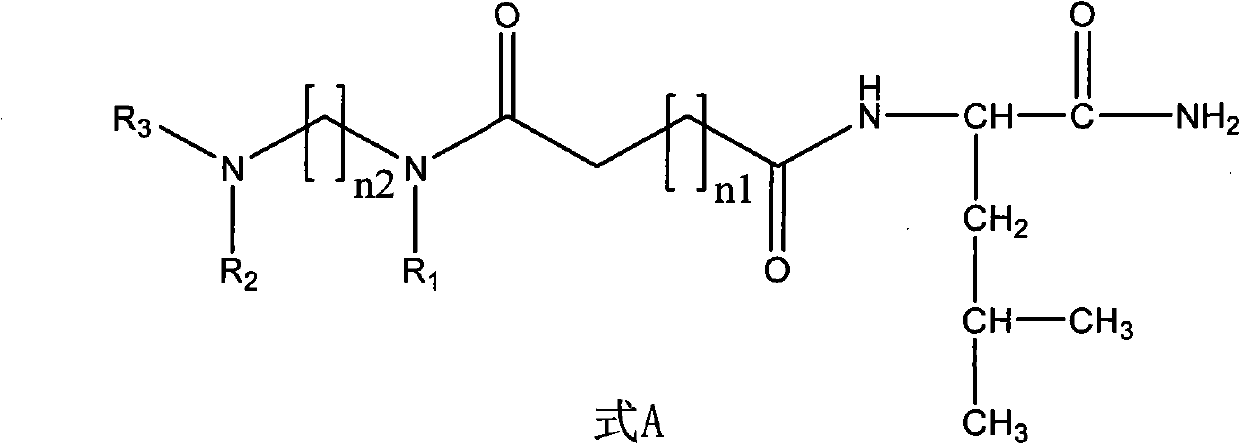
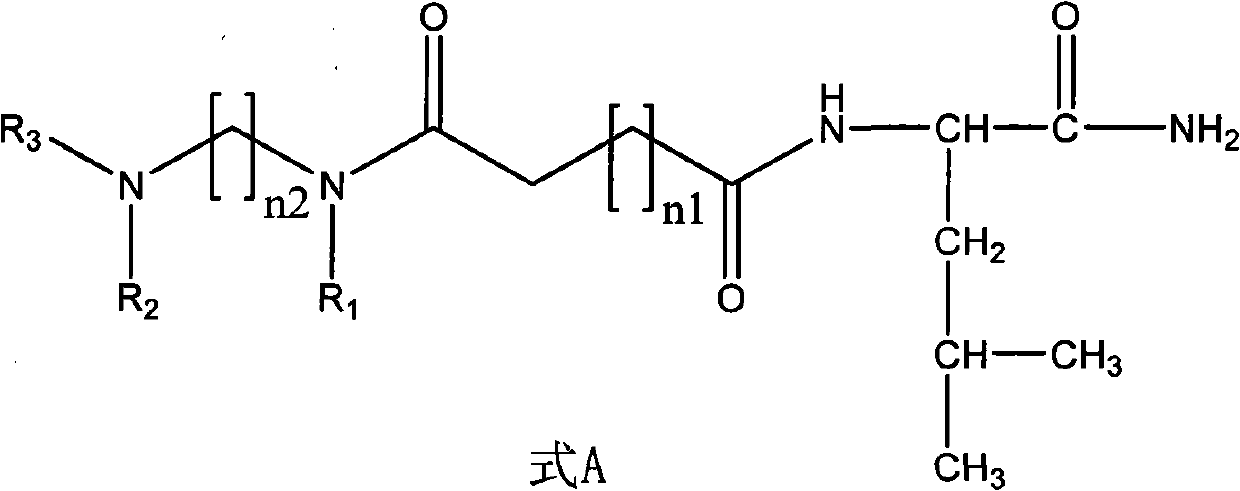
Non-peptide juvenile hormone synthetic inhibitor
A juvenile hormone and inhibitor technology, which is applied in the field of non-peptide juvenile hormone synthesis inhibitors, can solve the problems of complex structure, unsuitability for development and high synthesis cost, and achieves low cost, good development and application prospects, and novel structure. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1、A1
[0027] The synthesis of preparation embodiment 1, A1 compound
[0028] (1) Synthesis of non-peptide fragment 1: Add ethylenediamine (1 times the amount) dropwise into an ethanol solution of benzaldehyde (2.2 times the amount), stir at room temperature for 20 minutes, then add sodium borohydride ( 2.2 times the amount), slowly warming up to room temperature. Water and dichloromethane were added to the reaction solution, and the organic layer was extracted and dried. The dried substance was dissolved in dichloromethane, succinic anhydride (2.2 times the amount) was added and stirred at room temperature overnight, and the non-peptide fragment 1 was obtained by filtration and purification. The specific structural formula is:
[0029]
[0030] (2) Resin activation: Weigh 200 mg Rink Amide-AM resin, swell and activate it with 15 ml DCM for 3 h, add 20% piperidine DMF solution to cut for 20 min, and monitor the reaction with Kaiser’s reagent.
[0031] (3) Connecting to Leu: add...
preparation Embodiment 2、A2
[0037] The synthesis of preparation embodiment 2, A2 compound
[0038] (1) Synthesis of non-peptide fragment 1: Add ethylenediamine (1 times the amount) dropwise into an ethanol solution of benzaldehyde (2.2 times the amount), stir at room temperature for 20 minutes, then add sodium borohydride ( 2.2 times the amount), slowly warming up to room temperature. Water and dichloromethane were added to the reaction solution, and the organic layer was extracted and dried. The dried substance was dissolved in dichloromethane, succinic anhydride (2.2 times the amount) was added and stirred at room temperature overnight, and the non-peptide fragment 1 was obtained by filtration and purification. The structural formula of non-peptide fragment 1 is:
[0039]
[0040] (2) Resin activation: Weigh 200 mg Rink Amide-AM resin, swell and activate it with 15 ml DCM for 3 h, add 20% piperidine DMF solution to cut for 20 min, and monitor the reaction with Kaiser’s reagent.
[0041] (3) Conne...
preparation Embodiment 3、A3
[0045] The synthesis of preparation embodiment 3, A3 compound
[0046] (1) Synthesis of non-peptide fragment 2: 1,4-butanediamine (1 times the amount) was dropped into the ethanol solution of benzaldehyde (2.2 times the amount), stirred at room temperature for 20 minutes, and then added in batches when cooled to 0 degrees Sodium borohydride (2.2 times the amount), slowly warming up to room temperature. Water and dichloromethane were added to the reaction solution, and the organic layer was extracted and dried. The dried substance was dissolved in dichloromethane, succinic anhydride (2.2 times the amount) was added and stirred at room temperature overnight, and the non-peptide fragment 2 was obtained by filtration and purification. The structural formula of non-peptide fragment 2 is:
[0047]
[0048] (2) Resin activation: Weigh 200 mg Rink Amide-AM resin, swell and activate it with 15 ml DCM for 3 h, add 20% piperidine DMF solution to cut for 20 min, and monitor the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com