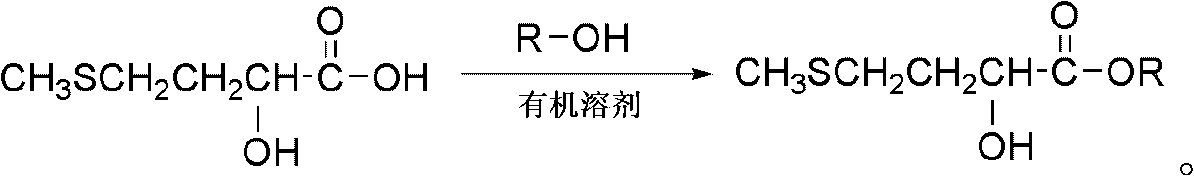
Preparation method of 2-hydroxy-4-methylthioalkyl butyrate
A technology of alkyl methylthiobutyrate and methylthiobutyric acid, which is applied in the field of preparation of alkyl butyrate, can solve the problems of cumbersome process, low yield, large dosage, etc. The effect of simple process
Inactive Publication Date: 2011-06-15
张维军
View PDF6 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Taking a broad view of the preparation technical schemes of the existing 2-hydroxyl-4-methylthiobutyrate alkyl esters, they can be roughly divided into three categories: the first category is to use 2-hydroxyl-4-methylthiobutyrate as raw material Preparation, the process of this preparation process is relatively loaded down with trivial details, and cost is higher, is unfavorable for industrialization, large-scale production; It is produced after esterification. The yield of this process is low, and the amount of concentrated sulfuric acid, organic solvent, water and other reaction media used in the process is relatively large, which not only increases the cost, but also makes it difficult to discharge waste liquid and waste gas. Solution, it does not belong to the production process of environmental protection and green, so the application prospect of this type of process is not promising; the third type is to directly carry out esterification with alcohol under the catalysis of concentrated sulfuric acid, and the yield of this method is relatively high. However, the purity of the product is not high enough, the ester content in the product can only reach 92%, and the product also contains a relatively high content of dimers and reaction raw materials
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a preparation method of an alkyl butyrate, and particularly discloses a preparation method of a 2-hydroxy-4-methylthioalkyl butyrate, which comprises the following steps: under the catalytic action of phosphoric acid, reacting 2-hydroxyl-4-methylthiobutyric acid and an alcohol, which are used as reaction materials, in an esterification mode; and separating and purifying the reaction products to obtain the 2-hydroxy-4-methylthioalkyl butyrate. The method disclosed by the invention has the advantages of low cost, simple technological process, high yield, high product purity and the like.
Description
The preparation method of 2-hydroxyl-4-methylthiobutyric acid alkyl ester technical field The invention relates to a method for preparing organic carboxylate, in particular to a method for preparing alkyl butyrate. Background technique Methionine hydroxy analogue (MHA-FA) is a liquid substance newly synthesized in recent years with the biological activity of methionine. In addition to the function of methionine, it also has the functions of acidifier. Although the liquid methionine hydroxyl analog has no amino group, it can still be synthesized into L-methionine in the body to participate in the metabolism in the body. Because it does not contain amino groups, deamination does not occur in metabolism. When methionine is metabolized in the body to form methionine, the free ammonia in the blood is used to increase nitrogen deposition in the body, improve nitrogen utilization efficiency, reduce nitrogen excretion, and reduce pollution of the environment. Although methionin...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C323/52C07C319/20
Inventor 张维军
Owner 张维军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com