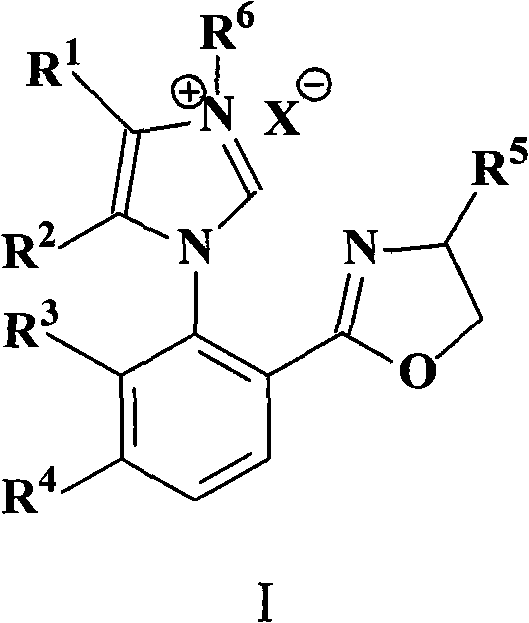
N-aryl axially chiral carbene-oxazoline compound and application thereof
A compound and axial chiral technology, applied in the field of N-aryl axial chiral carbene-oxazoline compounds, to achieve the effect of expanding design ideas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] (1) Under an ice bath, thionyl chloride was slowly added dropwise by 1mol in 10ml methanol solution of 0.2mol 1-hydroxyl-2-naphthoic acid, heated to reflux temperature after rising to room temperature, TLC (PE: EA(v / v) is 20:1, R f = 0.4) monitor the complete reaction; washing, drying, concentration, column chromatography (PE: EA (v / v) is 40: 1) to obtain compound IV-1; the yield is 69%;
[0047] 1 H NMR (CDCl 3 , 400MHz) δ3.98(s, 3H), 7.27(d, J=10Hz, 1H), 7.52(d, J=5Hz, 1H), 7.60(d, J=6Hz, 1H), 7.75-7.76(m , 2H), 8.42(d, J=4Hz, 1H), 11.99(s, 1H);
[0048] (2) 0.4mol pyridine was added to 0.2mol compound IV-1 in 8ml dichloromethane solution; Under ice bath, 0.6mol trifluoromethanesulfonic anhydride was slowly dripped into the solution, reacted at room temperature for three hours, TLC (PE : EA(v / v) is 10:1, R f =0.4) Monitor compound IV-1 reaction complete; After washing, drying, concentrating, column chromatography (eluent is PE: EA (v / v) is 40:1, 20:1 and 10:1) ob...
Embodiment 2
[0071] Preparation of palladium complex:
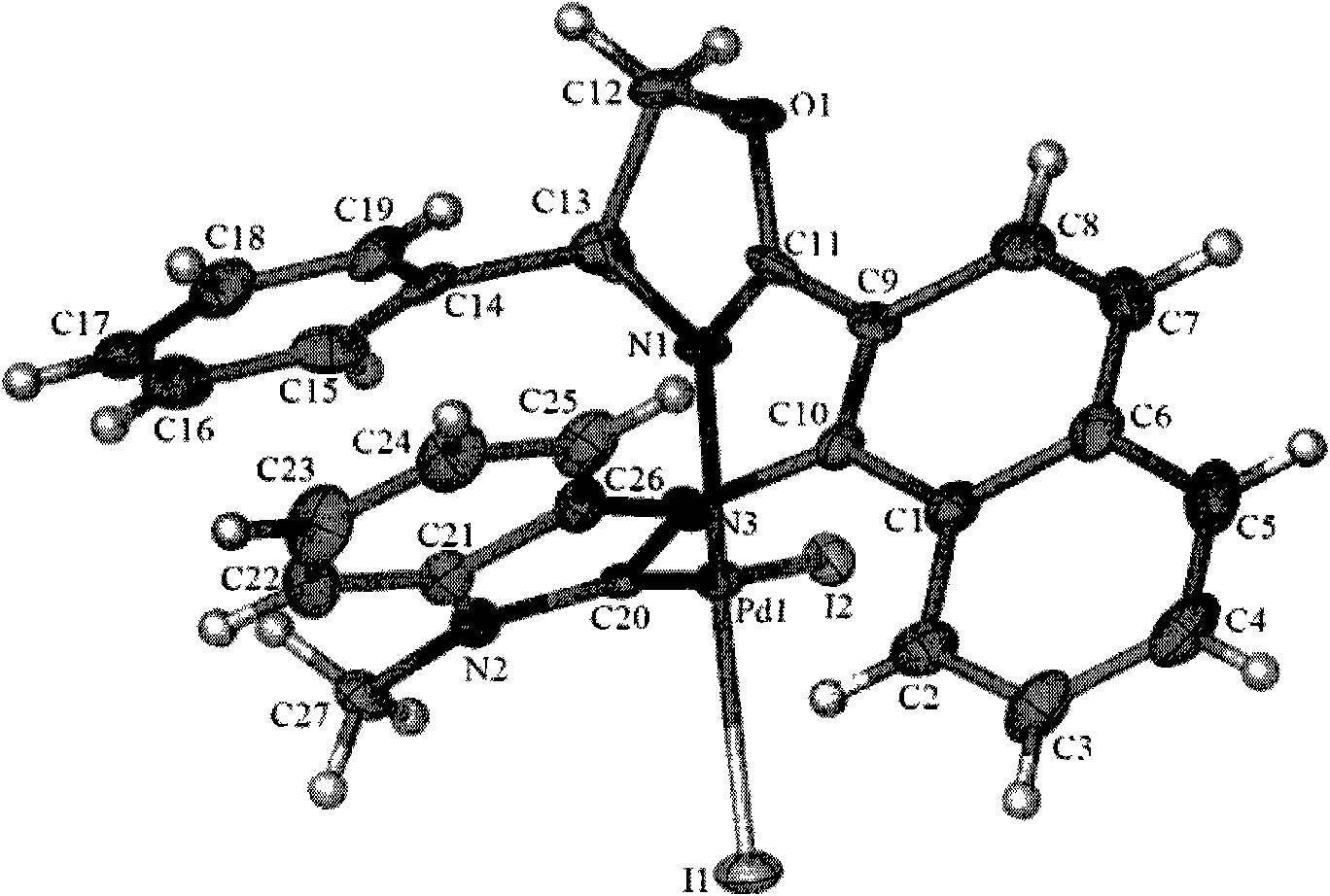
[0072] 8ml tetrahydrofuran was added to the reaction tube that 0.2mol compound Ia-1, 0.2mol palladium acetate and 0.4mol potassium iodide were housed, reflux reaction 12 hours, TLC (developing agent is dichloromethane (DCM), R f =0.5) It is monitored that the reaction of raw materials is complete, washed with water, dried, concentrated, column chromatography (eluent is PE: DCM (v / v)=6: 1 and DCM) to obtain compound IIa-Pd, its X-Ray figure Such as figure 1 As shown, the yield was 80%.
[0073]
[0074] Compound IIa-Pd
[0075] Compound IIa-Pd: 1 H NMR (400MHz, CDCl 3 , TMS) δ3.46 (s, 3H), 4.63 (dd, J 1 =6Hz,J 2 =10Hz, 1H), 5.06(t, J=10Hz, 1H), 6.13(dd, J 1 =6Hz,J 2 =10Hz, 1H), 6.64(d, J=8Hz, 1H), 6.92(d, J=7Hz, 2H), 7.14-7.23(m, 3H), 7.37-7.43(m, 4H), 7.49(t, J=8Hz, 1H), 7.70(t, J=8Hz, 1H), 7.96(d, J=8Hz, 1H), 8.11(d, J=8Hz, 1H), 8.29(d, J=9Hz, 1H) .
Embodiment 3
[0077] Preparation of gold complexes:
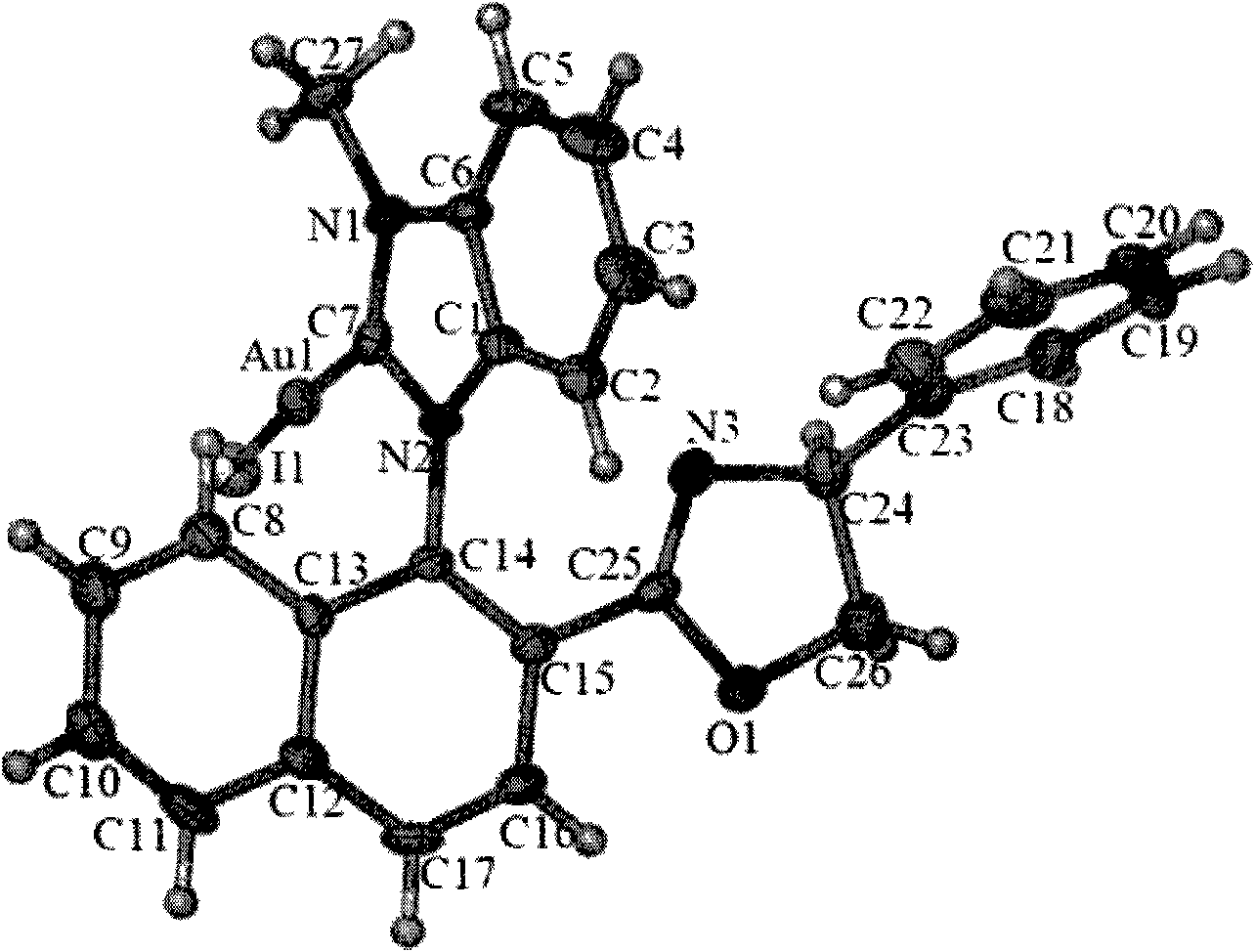
[0078] 8ml of acetonitrile was added to the reactor equipped with 0.1mol compound Ia-1 and compound Ib-1, 0.1mol dimethyl sulfide gold chloride and 0.2mol sodium acetate respectively, reacted at room temperature for 24 hours, TLC (developing agent PE: DCM (v / v) is 3:1, R f =0.25) to monitor that the reaction of the raw material is complete, after concentration, column chromatography (eluent is PE: DCM (v / v)=6:1 and 3:1) to obtain compound IIa-Au and compound IIb-Au; two The yields of the two configurations were 95% and 45%, respectively.
[0079]
[0080] Compound IIa-Au: 1 H NMR (400MHz, CDCl 3 , TMS) δ3.87 (t, J=9Hz, 1H), 4.16 (s, 3H), 4.45 (dd, J 1 =8Hz,J 2 =10Hz, 1H), 5.15(t, J=10Hz, 1H), 6.73-6.76(m, 2H), 6.89(d, J=8Hz, 1H), 7.16-7.17(m, 3H), 7.31-7.38( m, 2H), 7.47-7.54(m, 3H), 7.65(t, J=8Hz, 1H), 8.03(d, J=8Hz, 1H), 8.14-8.28(m, 2H), [α] 20 D +33.0 (c 0.2, CHCl 3 ); its X-Ray diagram is as follows figure 2 shown;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com