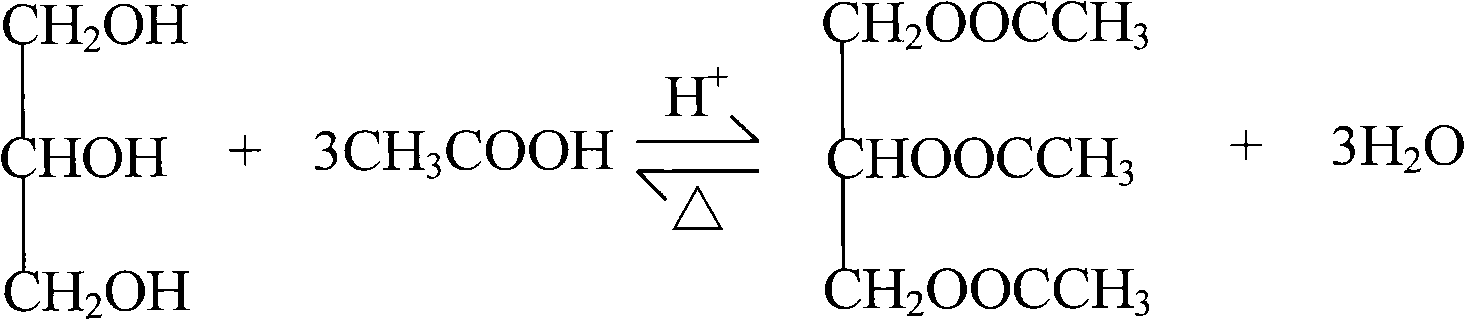Process and device for continuously producing glyceryl triacetate
A technology of glycerol triacetate and technology, which is applied in the field of continuous production of glycerol triacetate, can solve the problems of reducing the rate of esterification reaction, reducing the yield of final products, and increasing cost consumption, so as to avoid equipment corrosion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
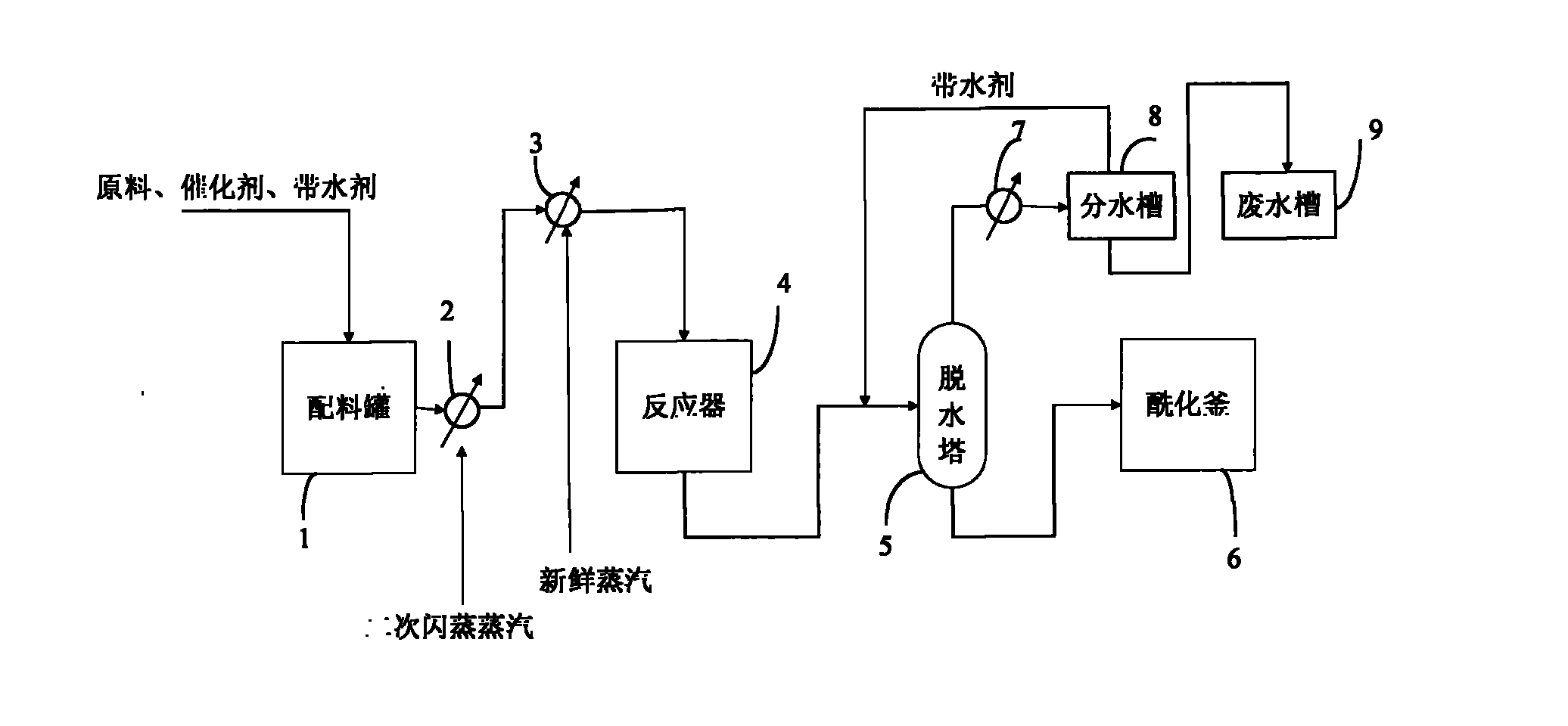
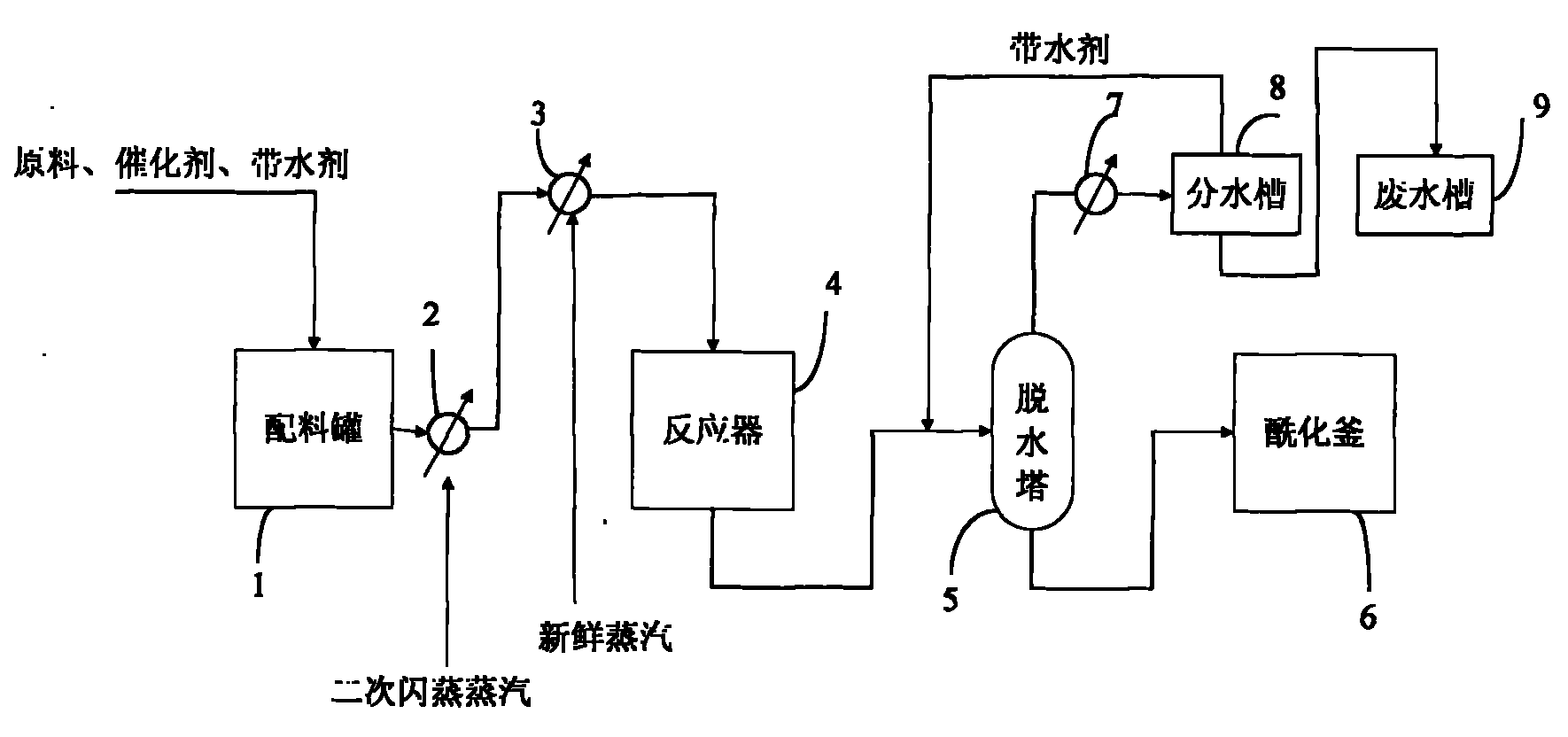
[0068] Put glycerin at 10000kg / 30h, acetic acid at 31000kg / 30h, ethyl acetate at 5000kg / 30h, and p-toluenesulfonic acid at 23kg / 30h into the batching tank, and send it into the reactor through a circulating pump.
[0069] The conditions in the control reactor are as follows:
[0070] Reaction temperature: 155°C; pressure: 0.28MPa; residence time: 20 hours and 20 hours later, the reacted mixture enters the dehydration tower, and the temperature at the top of the dehydration tower is controlled to be 86°C.
[0071] When there is no water removal at the top of the dehydration tower, the mixed product at the bottom of the dehydration tower is sent to the acylation kettle for processing, and the final product of the acylation kettle is intercepted at 21850kg / 30h.
[0072] According to the ratio of raw materials, the theoretical production of triacetin is 23700kg / 30h, the actual production of triacetin is 21850kg / 30h, and the yield reaches 92.2%.
Embodiment 2
[0074] Put glycerin at 15000kg / 40h, acetic acid at 48000kg / 40h, ethyl acetate at 8000kg / 40h, and p-toluenesulfonic acid at 50kg / 40h into the batching tank, and then into the reactor through a circulating pump.
[0075] The conditions in the control reactor are as follows:
[0076] Reaction temperature: 160°C; pressure: 0.31MPa; residence time: 25 hours
[0077] After 25 hours, the reacted mixture entered the dehydration tower, and the temperature at the top of the dehydration tower was controlled to be 87°C. When there is no water removal at the top of the dehydration tower, the mixed product at the bottom of the dehydration tower is sent to the acylation kettle for processing, and the final product of the acylation kettle is intercepted to be 32900kg / 40h.
[0078] According to the ratio of raw materials, the theoretical production of triacetin is 35540kg / 40h, the actual production of triacetin is 32900kg / 40h, and the yield reaches 92.6%.
Embodiment 3
[0080] Put glycerin at 20000kg / 40h, acetic acid at 64000kg / 40h, ethyl acetate at 9300kg / 40h, and p-toluenesulfonic acid at 93kg / 40h into the batching tank, and send it into the reactor through a circulating pump.
[0081] The conditions in the control reactor are as follows:
[0082] Reaction temperature: 165°C; pressure: 0.31MPa; residence time: 25 hours After 25 hours, the reacted mixture enters the dehydration tower, and the temperature at the top of the dehydration tower is controlled to be 88°C.
[0083] When there is no water removal at the top of the dehydration tower, the mixed product at the bottom of the dehydration tower is sent to the acylation kettle for processing, and the final product of the acylation kettle is intercepted to be 43970kg / 30h.
[0084] According to the ratio of raw materials, the theoretical production of triacetin is 47400kg / 40h, the actual production of triacetin is 43970kg / 40h, and the yield reaches 92.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com