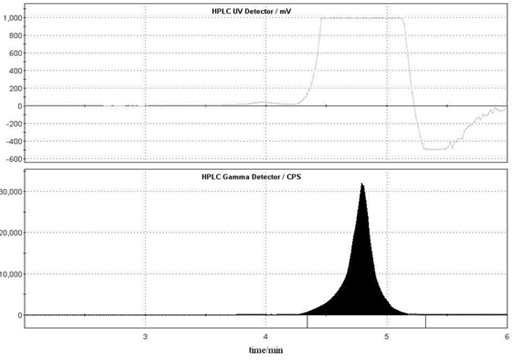
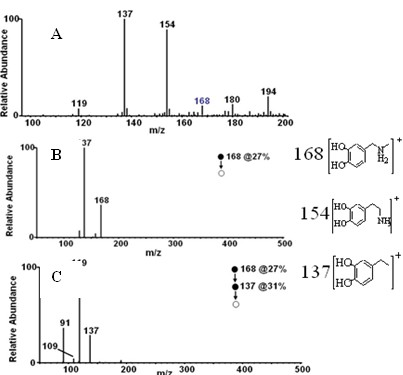
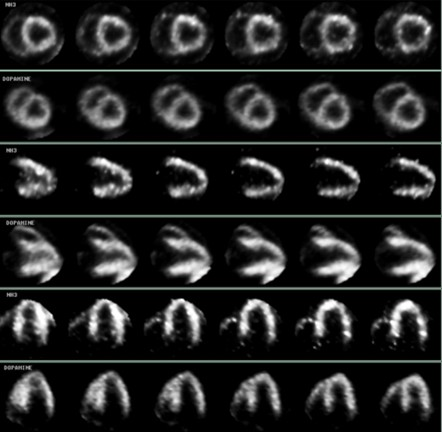
Use of carbon-11 marked N-methyldopamine hydrochloride in preparation of positron medicinal imaging agent
A technology of methyl dopamine and imaging agent, which is applied in the application field of carbon-11 labeling N-methyl dopamine and preparing positron drug imaging agent, which can solve the problems of insufficient research, high cost of electrophilic synthesis, and low yield Low-level problems, to achieve high-specificity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of carbon-11 labeled N-methyl dopamine
[0030] one. 11 C-N-CH 3 -Synthesis of Dopamine
[0031] 1. 11 C- 11 CH 3 Preparation of I
[0032] produced by the cyclotron 11 C-CO 2 It is transported into the chemical reactor and mixed with hydrogen, and reacted at 400°C under the action of Ni catalyst to form 11 CH 4 ; 11 CH 4 React with sublimated iodine at a high temperature of 730°C to generate carbon-11 labeled methyl iodide ( 11 CH 3 I), 11 CH 3 I enters the reaction bottle under the flow rate of 20 mL / min in the helium flow, and 0.4 mL of acetone is placed in the reaction bottle, and placed in the cold hydrazine of minus 20 degrees, finally obtaining 200mCi of carbon-11 methyl iodide acetone solution, wherein The chemical amount of methyl iodide is 2.88ug (when the carbon-11 methyl iodide acetone solution is 300mCi, the chemical amount of methyl iodide is 4.32ug):
[0033] .
[0034] 、 11 CH 3 I react fully with 3 mg (3-6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com