Water-soluble photostable silver (I) complex and preparation method and application thereof
A light-stable, water-soluble technology, applied in botanical equipment and methods, silver-organic compounds, applications, etc., to achieve mild conditions, high yields, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0019] Experimental example 1 Preparation of complexes:
[0020] 1 mmol 3,4,5,6-tetrafluorophthalic acid (0.238 g), 2 mmol silver nitrate (0.339 g), and 40 mL methanol and N,N-dimethylformamide (volume ratio 3:1 ), stirred at room temperature for 0.5-1 hour, filtered, and evaporated at room temperature for 6 days to obtain a colorless needle-like single crystal, which was then washed with 10 mL methanol and 10 mL ether, and dried naturally. The yield was 75%.
[0021] The main infrared absorption peak is 3364 cm-1 , 1641 cm -1 , 1600 cm -1 , 1573 cm -1 , 1487 cm -1 , 1444 cm -1 , 1413 cm -1 , 1335 cm -1 , 1218 cm -1 , 1151 cm -1 , 1117 cm -1 , 1069 cm -1 , 1037 cm -1 , 1095 cm -1 , 947 cm -1 , 853 cm -1 , 753 cm -1 , 697 cm -1 , 616 cm -1 .
experiment example 2
[0022] Experimental example 2 Preparation of complexes:
[0023] 1 mmol 3,4,5,6-tetrafluorophthalic acid (0.238 g), 2.2 mmol silver nitrate (0.373 g) and 40 mL methanol and N,N-dimethylformamide (volume ratio 3:1 ), stirred at room temperature for 0.5-1 hour, filtered, and evaporated at room temperature for 6 days to obtain a colorless needle-like single crystal, which was then washed with 10 mL methanol and 10 mL ether, and dried naturally. The yield is about 72%.
[0024] The main infrared absorption peak is 3364 cm -1 , 1641 cm -1 , 1600 cm -1 , 1573 cm -1 , 1487 cm -1 , 1444 cm -1 , 1413 cm -1 , 1335 cm -1 , 1218 cm -1 , 1151 cm -1 , 1117 cm -1 , 1069 cm -1 , 1037 cm -1 , 1095 cm -1 , 947 cm -1 , 853 cm -1 , 753 cm -1 , 697 cm -1 , 616 cm -1 .
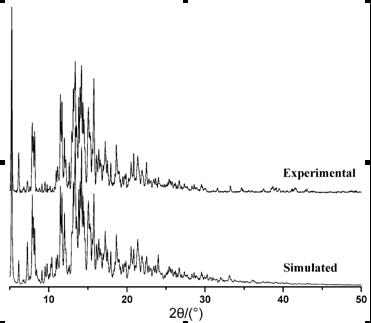
[0025] test one Characterization of complexes:
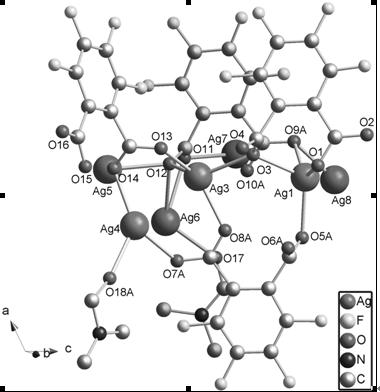
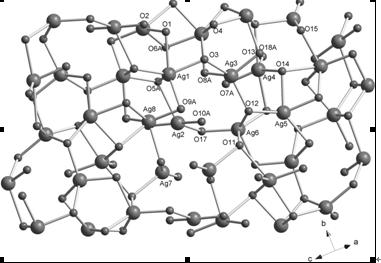
[0026] (1) Structure determination of complexes
[0027] The crystal structure was determined using a Bruker Apex II CCD diffractometer at 293(2) K with gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com