Quality control method for shenmai injection mass spectrum finger prints
A mass spectrometry fingerprint, Shenmai injection technology, applied in measurement devices, pharmaceutical formulations, plant raw materials and other directions, can solve the problem of insufficient expression of Ophiopogon japonicus components, achieve good technical support, high sensitivity, ensure integrity and exclusivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Analysis of Fingerprint I of Shenmai Injection
[0035] 1. Preparation of the test solution
[0036] Take an appropriate amount of Shenmai injection, centrifuge at 10,000 rpm for 10 min, and take the supernatant for later use;
[0037] 2. LC / MS Fingerprint of Shenmai Injection Ⅰ Chromatographic Conditions
[0038] Using C 18 Bonded Fast LC Column Edipse Plus C 18 (100 mm×4.6 mm i.d., 1.8 μm), the mobile phase is 0.06% formic acid aqueous solution A and acetonitrile B, the gradient elution program is: 0-10 min, 5-15% B; 10-25 min, 15-25% B; 25-30 min, 25-30% B; 30-45 min, 30-35% B; 45-50 min, 35-45% B; 50-70 min, 45-70% B; 70-75 min , 70-90% B, column temperature: 30°C, injection volume: 5μl;
[0039] 3. LC / MS Fingerprint Ⅰ Mass Spectrometry Conditions of Shenmai Injection
[0040]Atomization pressure: 45 psi; Nitrogen flow rate: 12 L / min; Capillary voltage: 3000 V; In-source pyrolysis voltage: 250 V; Nitrogen temperature: 250, 300 and 350°C; -1400;
...
Embodiment 2
[0042] Example 2 Analysis of Fingerprint I of Shenmai Injection
[0043] The preparation of the test solution and the chromatographic conditions are as in Example 1, except for the mass spectrometry conditions. Mass spectrometry conditions: Nitrogen temperature: 350°C; Nebulization pressure: 45 psi; Nitrogen flow rate: 12 L / min; In-source cracking voltage: 250 V; Capillary voltage: 2500, 3000, 3500 and 4000 V; Negative ion scanning detection mode, scanning m / z range: 200-1400;
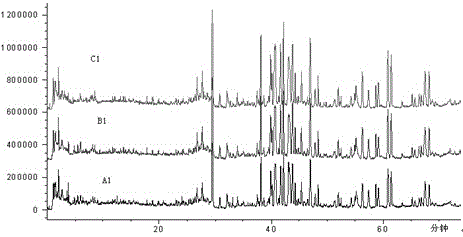
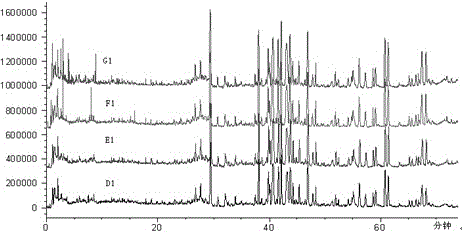
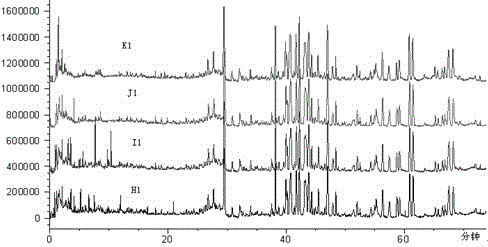
[0044] The results of the analysis are attached figure 2 , Total ion chromatogram of fingerprint Ⅰ of Shenmai injection at different capillary voltages.
Embodiment 3
[0045] Example 3 Analysis of Fingerprint I of Shenmai Injection
[0046] The preparation of the test solution and the chromatographic conditions are as in Example 1, except for the mass spectrometry conditions.
[0047] Mass spectrometry conditions: Nitrogen temperature: 350°C; Nebulization pressure: 45 psi; In-source cracking voltage: 250 V; Capillary voltage: 3500; Nitrogen flow rate: 9, 10, 11, 12 L / min; negative ion scanning detection mode, scanning m / Z range: 200-1400;
[0048] The results of the analysis are attached image 3 , total ion chromatogram of fingerprint Ⅰ of Shenmai injection at different nitrogen flow rates.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com