Construction and screening of rabies virus Evelyn-Rokitnicki-Abelseth (ERA) attenuated live vaccine candidate strain
A technology of rabies virus and attenuated vaccines, which is applied in antiviral agents, inactivated/attenuated, medical preparations containing active ingredients, etc., can solve the problems of unpredictability and long cycle of live attenuated vaccines, and achieve Save operating time, have a good technical platform, and improve rescue efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Construction and biological activity identification of rabies virus GP333 position R→E mutant strain
[0037] 1 Materials and methods
[0038] 1.1 Cells and viruses
[0039] Vero cells (African green monkey kidney cells, ATCC No.CCL-81): the culture medium is DMEM containing 10% fetal bovine serum; NA cells (neuroma cells, purchased from Changchun Academy of Military Medical Sciences): the culture medium is containing 10% MEM of fetal bovine serum; BHK-21 cells (suckling hamster kidney cells, ATCC No.CCL-10): the medium is DMEM containing 5% fetal bovine serum; the rabies virus vaccine strain used in the test is ERA strain vaccine (purchased from China Veterinary Drug Control Institute, AV61), the standard strain of rabies virus CVS used in the virus neutralization experiment was purchased from Changchun Academy of Military Medical Sciences.
[0040] 1.2 Main reagents and instruments
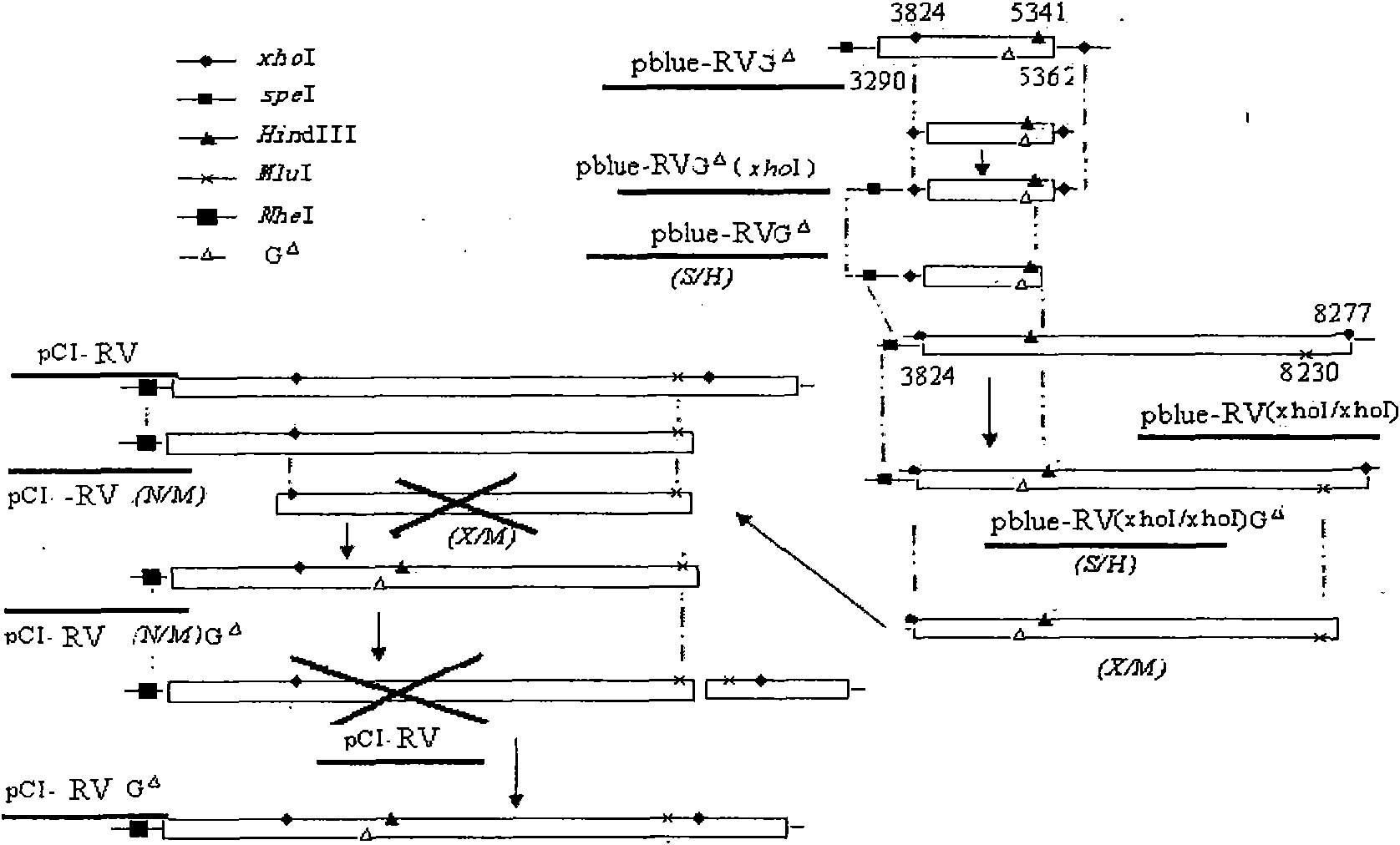
[0041] PrimerSTAR HS DNA Polymerase, T 4 DNA ligase and restriction en...
Embodiment 2
[0078] Example 2 Pathogenicity of rabies virus rRV-G333R / E mutant strain
[0079] Unless otherwise specified, the materials and methods of this example are the same as Example 1.
[0080] Pathogenicity detection of rRV-G333R / E mutant strain
[0081] Rabies virus ERA wild-type control strain (10 6 PFU / mL), rRV-G333R / E mutant strain (10 7 PFU / mL) stock solution was used to inoculate suckling mice and adult Balb / c mice (3 weeks old, 10-13g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. 10 mice after inoculation were raised in negative pressure cages with high-efficiency filtration devices, observed daily until 28 days after inoculation, and recorded the number of survivors. The mice that died within 4 days after inoculation were considered non-specific deaths and were not counted.
[0082] Results: Intracerebral inoculation of rabies virus ERA wild-type control strain and rRV-G333R / E mutant strain in suckling mice were all fatal. All the suckli...
Embodiment 3
[0083] Example 3 Immunogenicity of rabies virus rRV-G333R / E mutant strain
[0084] Unless otherwise specified, the materials and methods of this example are the same as Example 1.
[0085] Detection of immunogenicity of rRV-G333R / E mutant strain
[0086] 1. Immunogenicity of rRV-G333R / E mutant strain to mice
[0087] Rabies virus ERA wild-type control strain (10 6 PFU / mL), rRV-G333R / E mutant strain (10 7 PFU / mL) stock solution was diluted to 10 6 PFU / mL and 2*10 6 PFU / mL, artificially immunize 5-week-old adult Balb / c mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) with 100 μL / only by intramuscular injection. In the non-immune control group, 21 days after immunization, the virus of the same generation was used to boost immunization by intramuscular injection with the same dose. Three weeks after the booster immunization, blood was collected to determine the serum neutralizing antibody titer.
[0088] 2. Immunogenicity of rRV-G333R / E mu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com