IAPP (Islet Amyloid Polypeptide) analog injection with better stability
An analogue and stable technology, applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of poor stability and unfavorable patient acceptance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
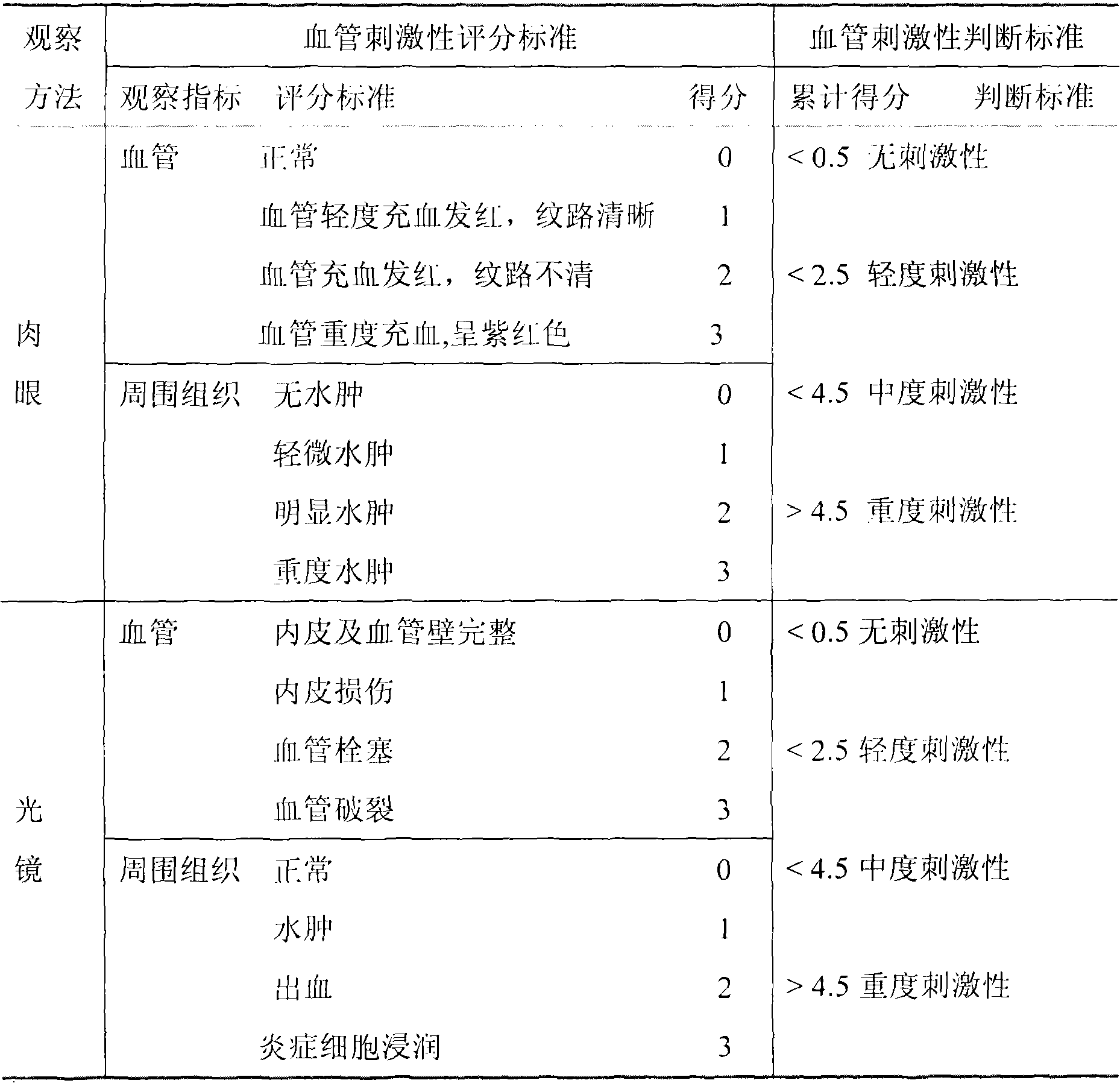
Image
Examples
Embodiment 1
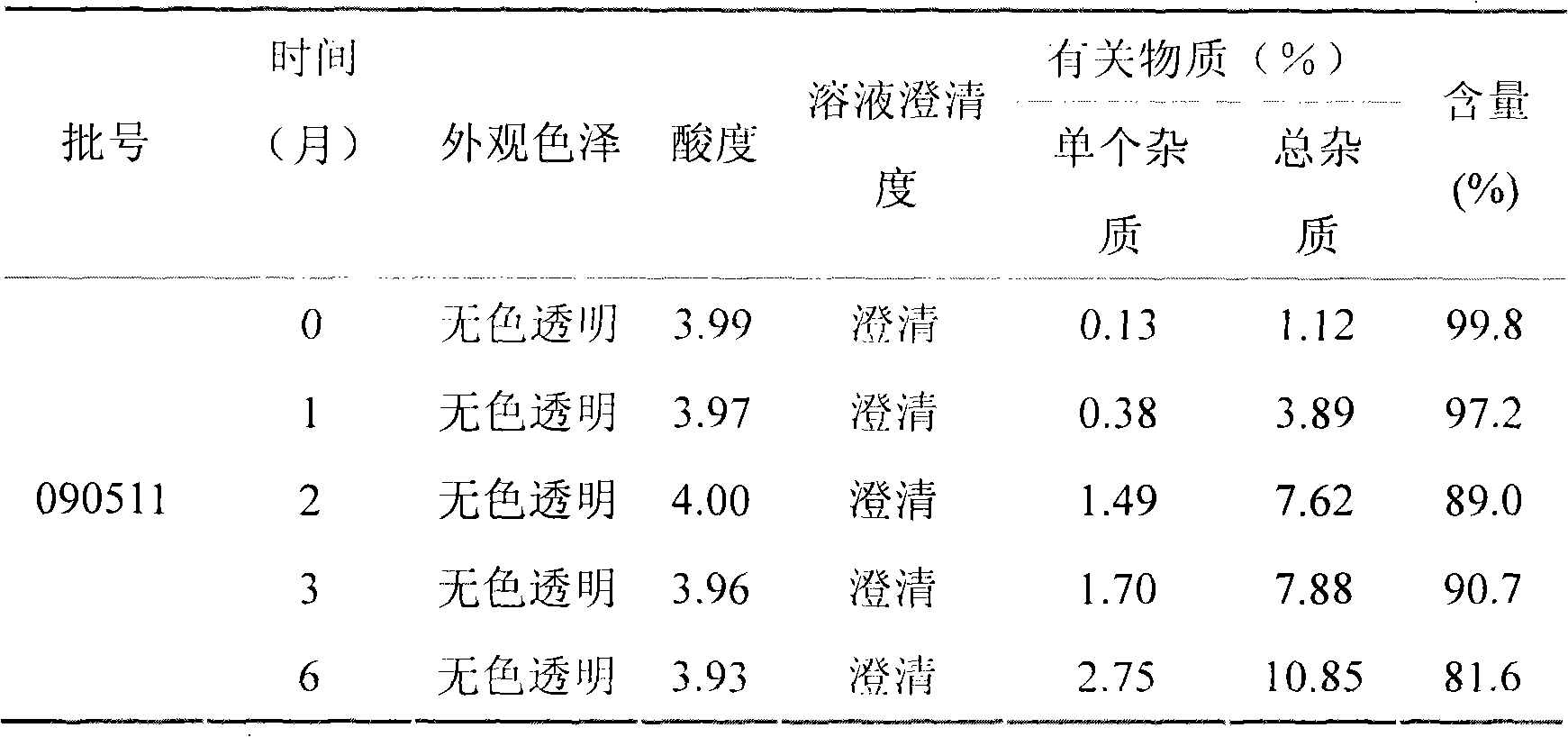
[0022] Pramlintide acetate (0.60 mg / ml as pramlintide), 2.25 mg / ml m-cresol, 43.00 mg / ml mannitol, 0.1% w / v sodium bisulfite, 2% w / v benzyl alcohol, with Acetic acid and sodium acetate regulate pH to be 4.0, and its preparation process is as follows:
[0023] Weigh pramlintide acetate (3g in terms of pramlintide), 11.25g m-cresol, 215.0g mannitol, 5.0g sodium bisulfite, 100.0g benzyl alcohol, dissolve completely with 80% water for injection of prescription quantity, and use acetic acid Adjust the pH to 4.0 with sodium acetate, and add water for injection to 5000ml. Before filtering, add 5g of activated carbon to the injection, absorb pyrogen for 30 minutes under stirring, decarbonize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane, filled in a 7ml sterilized vial (5ml / bottle), and sealed with a cap. Make each vial equivalent to 3 mg pramlintide.
[0024] Prepare 1000 bottles...
Embodiment 2
[0056] Pramlintide acetate (0.60 mg / ml as pramlintide), 2.25 mg / ml m-cresol, 43.00 mg / ml mannitol, 0.1% w / v sodium metabisulfite, 0.4% w / v chlorobutanol, Regulate pH with acetic acid and 1 sodium acetate to be 4.0, and its preparation process is as follows:
[0057] Weigh pramlintide acetate (3g in pramlintide), 11.25g m-cresol, 215.0g mannitol, 5.0g sodium metabisulfite, 20.0g chlorobutanol, dissolve completely with prescription amount 80% water for injection, use Adjust the pH to 4.0 with acetic acid and sodium acetate, and add water for injection to 5000ml. Before filtering, add 5g of activated carbon to the injection, absorb pyrogen for 30 minutes under stirring, decarbonize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane, filled in a 7ml sterilized vial (5ml / bottle), and sealed with a cap. Make each vial equivalent to 3 mg pramlintide.
[0058] Prepare 1000 bottles of p...
Embodiment 3
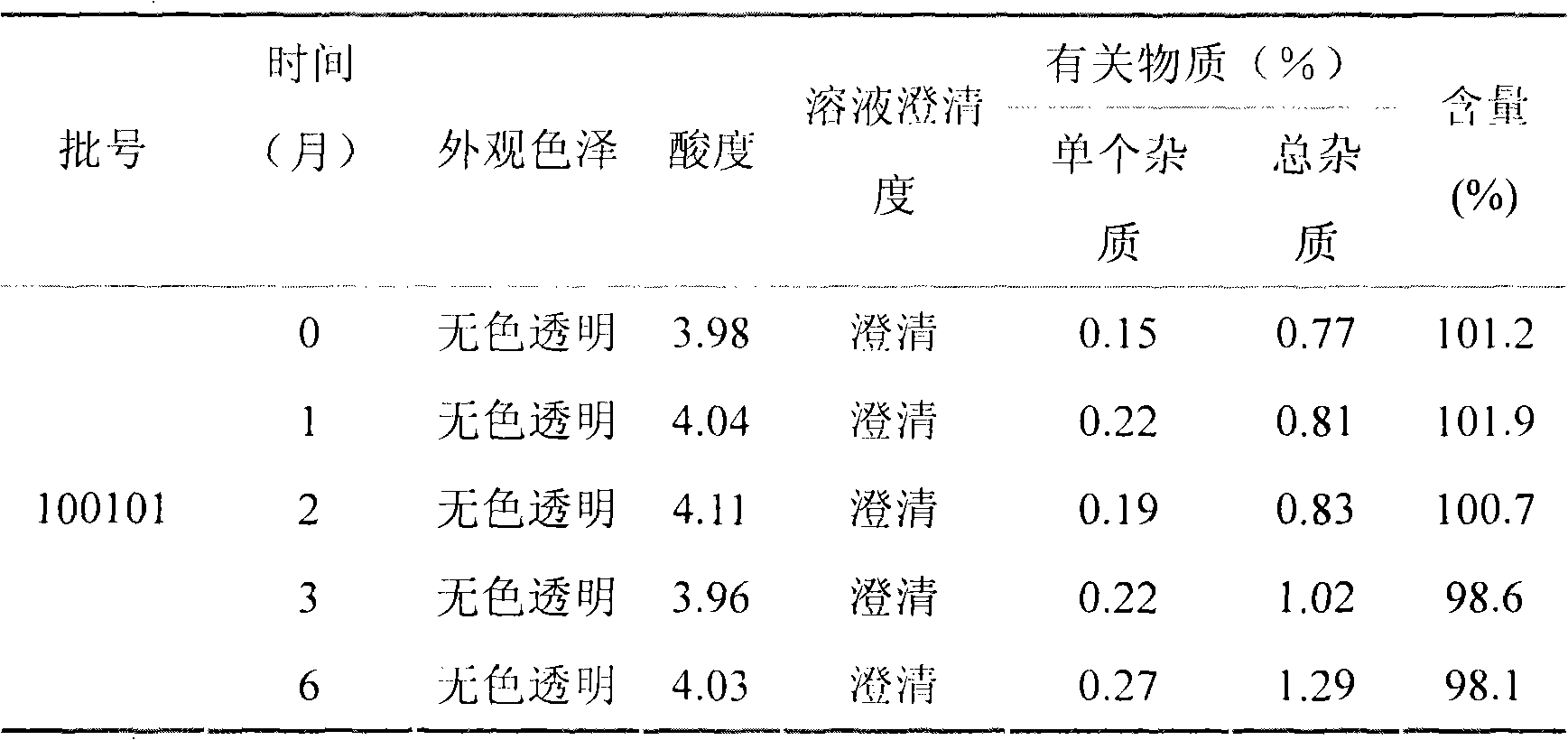
[0090] Pramlintide acetate (1.00 mg / ml as pramlintide), 2.25 mg / ml m-cresol, 5% w / v glucose, 0.1% w / v ascorbic acid, 2% w / v benzyl alcohol, with acetic acid and acetic acid Sodium regulates pH to be 4.0, and its preparation process is as follows:
[0091] Weigh pramlintide acetate (1.5g in terms of pramlintide), 3.38g m-cresol, 75.0g glucose, 1.5g ascorbic acid, 30.0g benzyl alcohol, dissolve completely with prescription amount 80% water for injection, and use acetic acid and sodium acetate Adjust the pH to 4.0, add water for injection to 1500ml. Before filtering, add 1.5g of activated carbon to the injection, absorb pyrogen for 30 minutes under stirring, decarbonize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane, filled in a 3 ml sterilized vial (1.5 ml / bottle), and sealed with a cap. Make each vial equivalent to 1.5 mg pramlintide.
[0092] Prepare 1000 bottles of pramlin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com