Method for separating boron isotopes by simulated moving bed chromatography
A simulated moving bed and chromatographic separation technology, which is applied in the field of stable boron isotope separation by combining ion exchange chromatography and simulated moving bed technology, can solve problems such as no public reports, and achieve the effect of reducing production costs and high separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
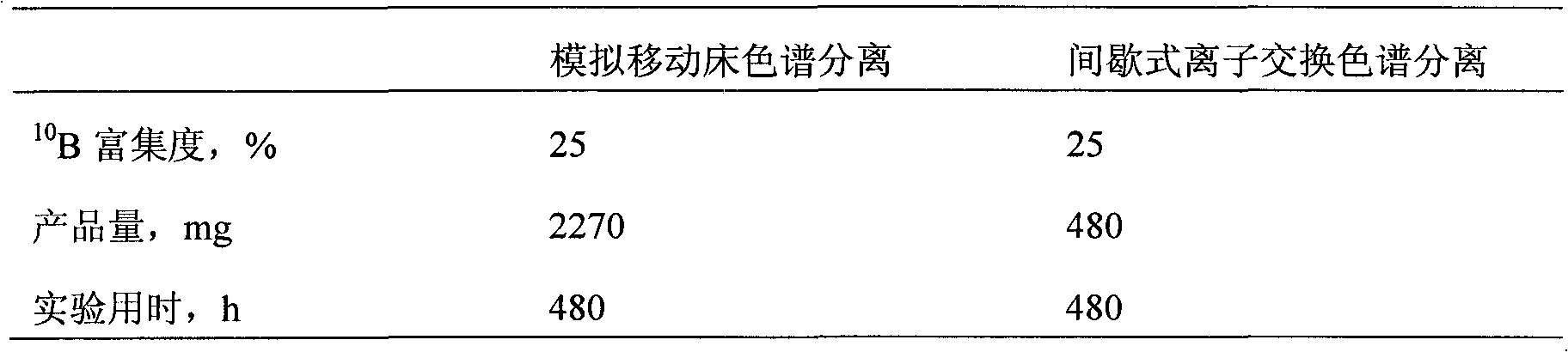
Examples
Embodiment 1
[0034] Step 1: Prepare boric acid aqueous solution
[0035] Take 3 g of boric acid solids with an electronic balance, put them in a beaker, add distilled water, and stir to dissolve; pour the solution into a 500ml volumetric flask, wash the beaker with distilled water for 3 times, pour the lotion into the volumetric flask, shake at a constant volume, and prepare 6g / L boric acid solution;
[0036] Step 2: Filter and remove impurities
[0037] Filter the boric acid solution with a 0.45 μm filter membrane to remove impurities in the solution, and then perform ultrasonic degassing on the filtered boric acid aqueous solution for 10 minutes to obtain the sample solution of the simulated moving bed. The simulated moving bed is composed of 8 chromatographic columns with a ratio of height to diameter of 10:1; the 8 chromatographic columns are divided into 4 zones, each zone is composed of 2 chromatographic columns; the chromatographic column filler is DIAN with an average particle siz...
Embodiment 2
[0041] Step 1: Prepare boric acid aqueous solution
[0042]Take 15g of boric acid solids with an electronic balance and place them in a beaker, add distilled water, and stir to dissolve; pour the solution into a 500ml volumetric flask, wash the beaker with distilled water for 3 times, pour the lotion into the volumetric flask, shake at a constant volume, and prepare 30g / L boric acid solution;
[0043] Step 2: Filter and remove impurities
[0044] Filter the boric acid solution with a 0.45 μm filter membrane to remove impurities in the solution, and then perform ultrasonic degassing on the filtered boric acid aqueous solution for 12 minutes to obtain the sample solution of the simulated moving bed. The simulated moving bed is composed of 8 chromatographic columns, the height-to-diameter ratio of the chromatographic columns is 10:1, and the 8 chromatographic columns are divided into 3 areas, of which the II area is composed of 4 chromatographic columns, and the I and III areas ...
Embodiment 3
[0048] Step 1: Prepare boric acid aqueous solution
[0049] Take 6 g of boric acid solids with an electronic balance, put them in a beaker, add distilled water, and stir to dissolve; pour the solution into a 500ml volumetric flask, wash the beaker twice with distilled water, pour the lotion into the volumetric flask, shake at a constant volume, and prepare 12g / L boric acid solution;
[0050] Step 2: Filter and remove impurities
[0051] Filter the boric acid solution with a 0.45 μm filter membrane to remove impurities in the solution, and then perform ultrasonic degassing on the filtered boric acid aqueous solution for 15 minutes to obtain the sample solution of the simulated moving bed. The simulated moving bed is composed of 12 chromatographic columns, which are divided into 4 areas, of which the II area is composed of 6 chromatographic columns, and the I, III, and IV areas are each composed of 2 chromatographic columns; the average particle size of the chromatographic colu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com