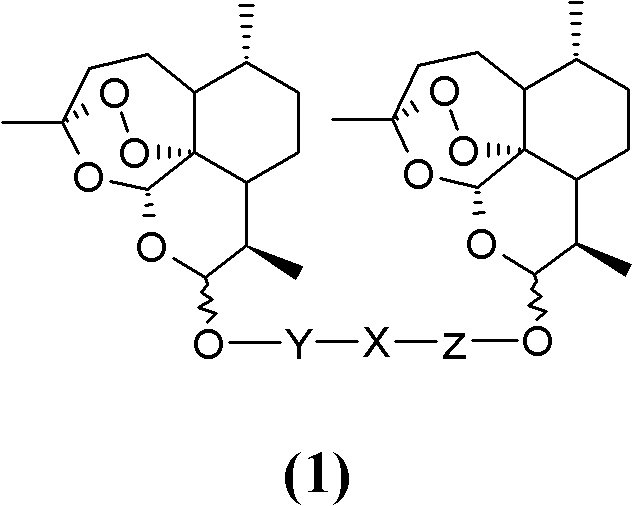
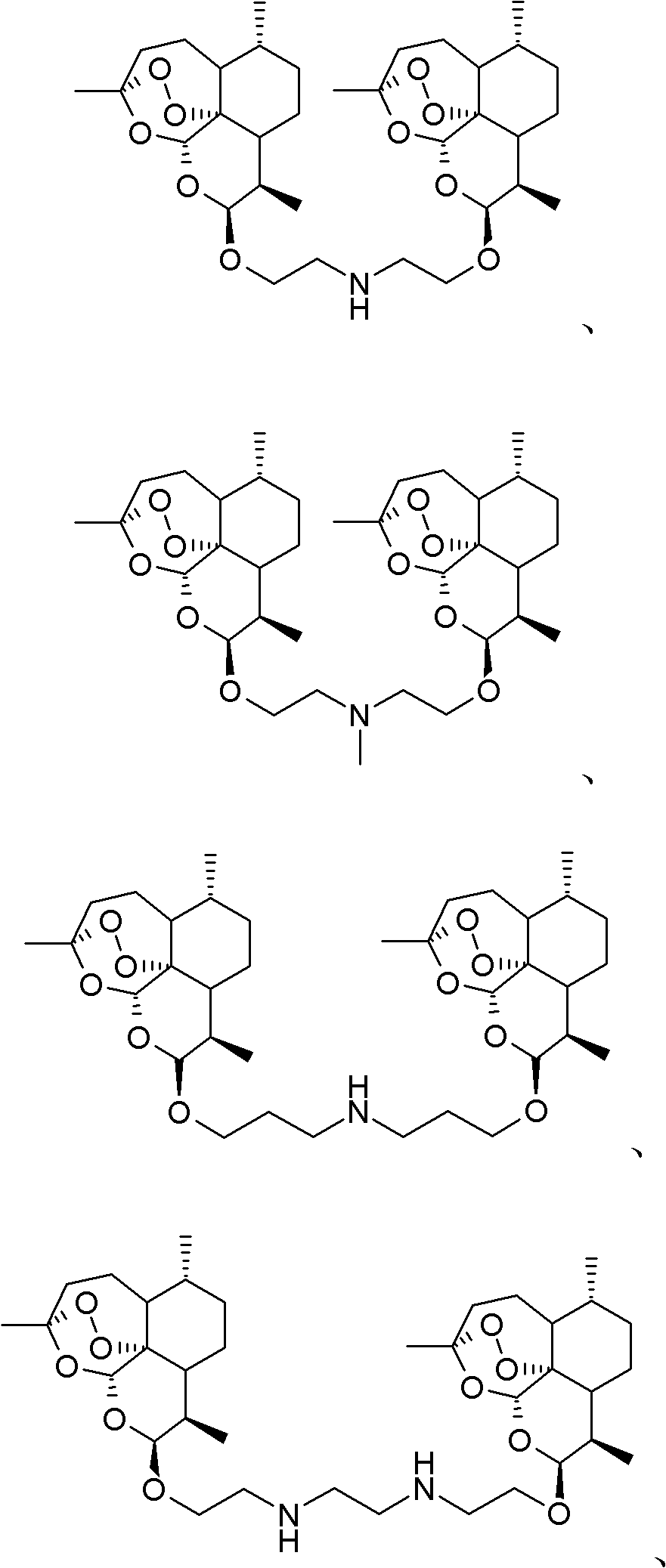
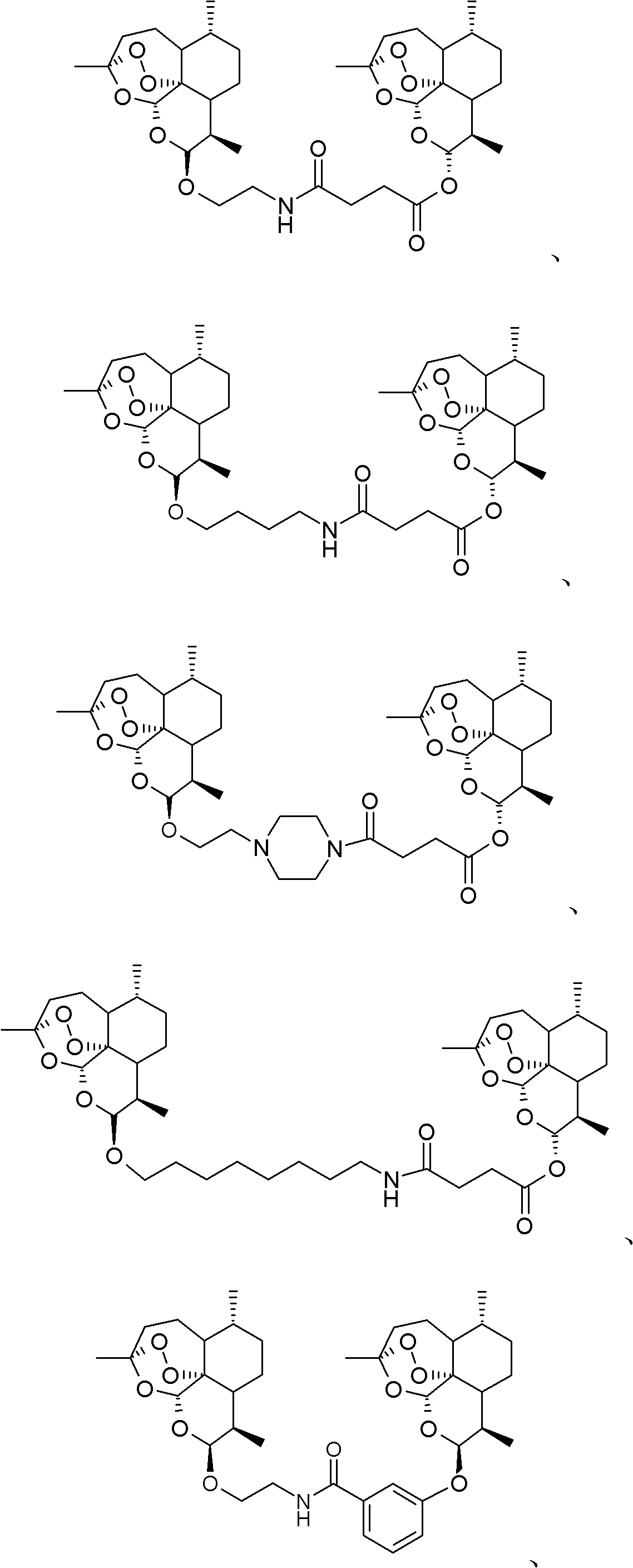
Nitrogen-atom-containing arteannuin dimers, and preparation method and application thereof
A technology of artemisinin and nitrogen atoms, applied to medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve problems such as serious side effects and poor quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1 (method A)
[0080]
[0081] First condense dihydroartemisinin and ethylene glycol in the presence of boron trifluoride ether complex to generate β-hydroxyarteether, and then react it with p-toluenesulfonyl chloride to obtain p-toluenesulfonate of β-hydroxyarteether Ester (compound 2, Y=C 2 h 4 ), white crystals, melting point 96-98°C.
[0082] Compound 2 (Y=C 2 h 4 , 1.54g) was dissolved in dimethylformamide (10mL), then ammonia water (0.5mL) was added, stirred and heated to 40-50°C, and reacted for about 20h. After TLC detected that the raw material spots basically disappeared, the reaction solution was poured into ice water, extracted repeatedly with ethyl acetate, the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the residue was subjected to column chromatography (silica gel, the eluent was a mixed solvent of ethyl acetate / petroleum ether...
Embodiment 2
[0090] Embodiment 2 (method A)
[0091]
[0092] First, dihydroartemisinin and propylene glycol are condensed in the presence of boron trifluoride ether complex to generate β-hydroxyartemisin, and then it is reacted with p-toluenesulfonyl chloride to obtain p-toluenesulfonate of β-hydroxyartemisinin Ester (compound 2, Y=C 3 h 6 ).
[0093] The p-toluenesulfonate (compound 2, Y=C 3 h 6 , 2.05g) and ammonia water (3.4mL) as raw materials, the reaction conditions and post-treatment method were the same as in Example 1 to obtain 0.9g of yellow oil, with a yield of 67%.
[0094] Using a method similar to that of Example 1, the melting point of the maleate salt (code name SM 1046) of the above yellow oil was 143-145°C; the melting point of succinate (code name SM 1048) was 136-138°C. Citrate (code SM 1047) melting point: 132-134°C.
[0095] 1 HNMR (free base, 300MHz, CDCl 3 )δ: 5.38(s, 2H), 4.77(d, J=4.2Hz, 2H), 3.90(m, 2H), 3.45(m, 2H), 2.72(t, J=7.2Hz, 4H), 1.43( s, 6H...
Embodiment 3
[0099] Embodiment 3 (method D)
[0100]
[0101] The p-toluenesulfonate of β-hydroxyarteether (compound 2, Y=C 2 h 4 , 4.8g) was reacted with methylamine ethanol solution, stirred and heated to 40-50°C, and reacted for about 20h. After TLC detected that the raw material spots basically disappeared, the reaction solution was concentrated, the residue was extracted with ethyl acetate several times, the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography (silica gel, the eluent was a mixed solvent of ethyl acetate / petroleum ether / triethylamine) to obtain 2.6 g of a light yellow oil, which was 7, Y=C 2 h 4 , R=CH 3 ).
[0102] The above product is then combined with p-toluenesulfonate of hydroxyarteether (compound 11, Z=C 2 h 4 , 1.9g) was dissolved in dimethylformamide (20mL), stirred and heated to 50°C in the prese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com