Preparation method of chiral warfarin and chiral warfarin derivatives
A derivative and chiral technology, applied in the field of preparation of coumarin anticoagulants - chiral warfarin and its derivatives, can solve the problem of weakening effect, increasing blood coagulation effect, and failing to obtain good experimental results, etc. problem, to achieve the effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
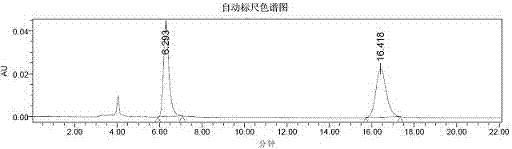
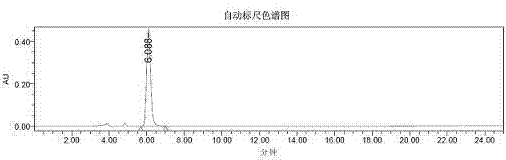
Image
Examples
Embodiment 1
[0043] 1) Preparation of intermediate substituted benzylidene acetone. Reaction formula:
[0044]
[0045] Steps:
[0046] In a 50ml single-necked round bottom flask, add benzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separation and purification (distillation under reduced pressure) gave a viscous oily liquid, and after cooling, a pale yellow solid—benzylidene acetone (150-160°C / 3333Pa (7mmHg)) was obtained.
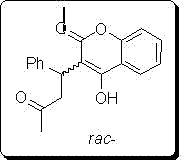
[0047] 2) Preparation of chiral warfarin. Reaction formula:
[0048]
[0049] Steps:
[0050] 10ml reaction test tube, add 4-hydroxycoumarin (0.1mmol), benzylidene acetone (0.12mmol), chiral primary amine catalyst (5mmol%), lithium perchlorate (5mmol%), Acetic acid (1mmol, 10eq), and finally 2ml of 1,4-dioxane was added. Stirring at room temperature for 24h, adding water to ...
Embodiment 2
[0061] 1) the preparation of intermediate product 4-methylbenzylidene acetone, reaction formula:
[0062]
[0063] Steps:
[0064] In a 50ml single-necked round bottom flask, add 4-methylbenzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separation and purification (distillation under reduced pressure) gave a viscous oily liquid, and after cooling, a pale yellow solid—benzylidene acetone (150-160°C / 3333Pa (7mmHg)) was obtained.
[0065] 2) Preparation of chiral warfarin derivatives, reaction formula:
[0066]
[0067] Steps:
[0068] 10ml reaction test tube, add 4-hydroxycoumarin (0.1621g, 0.1mmol, 1eq), intermediate product 4-methylbenzylideneacetone (0.220g, 0.12mmol, 1.2eq), chiral primary amine catalyst at one time (5mmol%), lithium perchlorate (5mmol%), acetic acid (1mmol, 10eq), an...
Embodiment 3
[0070] 1) Preparation of intermediate product 2-methoxybenzylideneacetone. Reaction formula:
[0071]
[0072] Steps:
[0073] In a 50ml single-necked round bottom flask, add 2-methoxybenzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separation and purification (distillation under reduced pressure) of the distillate gives a viscous oily liquid, and after cooling, a pale yellow solid—benzylidene acetone (150-160°C / 3333Pa (7mmHg))
[0074] 2) Preparation of chiral warfarin derivatives, reaction formula:
[0075]
[0076] (3);
[0077] Steps:
[0078] 10ml of reaction test tube, one-time addition of 4-hydroxycoumarin (0.1mmol), intermediate product 2-methoxybenzylidene acetone (0.12mmol), chiral primary amine catalyst (5mmol%), lithium perchlorate ( 5mmol%), acetic acid (1mmol, 10eq), and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com