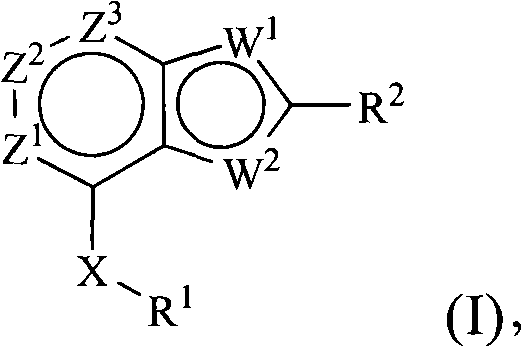
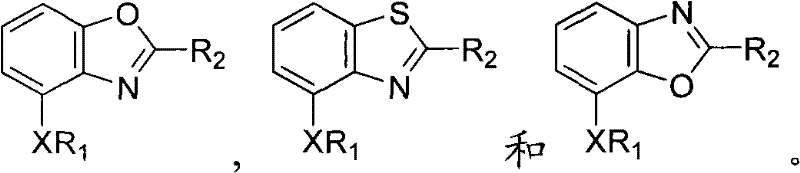
Benzoxazoles, benzthiazoles and related analogs as sirtuin modulators
A compound and alkyl technology, applied in the direction of drug combination, antiviral agent, active ingredient of heterocyclic compound, etc., can solve the problem of reducing and prolonging the life span of wild-type cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0292] Example 1. N-(2-(3-(trifluoromethoxy)phenyl)benzo[d] Synthesis of oxazol-4-yl)thiazole-4-carboxamide (compound 104):
[0293] Step 1) 4-nitro-2-(3-(trifluoromethoxy)phenyl)benzo[d] Preparation of azole (2):
[0294]
[0295] 2-Amino-3-nitrophenol (1; 2.00 g, 0.0130 mol) was dissolved together with 3-trifluoromethoxybenzoic acid (50; 2.67 g. 0.0130 mol) in 10 mL of polyphosphoric acid (PPA). The reaction mixture was stirred at 165°C for 3 hours. It was then cooled to about 100°C and carefully poured into 300 mL of water. Sufficient solid NaOH was added to bring pH=6. The resulting solid was collected by filtration, washed with water, and dried to give 800 mg of 4-nitro-2-(3-(trifluoromethoxy)phenyl)benzo[d] Azole 2, as light brown solid. MS(ESI) calculated value C 14 h 7 f 3 N 2 o 4 : 324.04; measured value: 325 [M+H]. Other compounds shown in Table 1 were prepared by using the appropriate carboxylic acids.
[0296] Step 2) 2-(3-(trifluoromethoxy)pheny...
Embodiment 2
[0303] Example 2. N-(thiazol-2-yl)-2-(2-(trifluoromethyl)phenyl)benzo[d] Synthesis of azole-4-carboxamide (compound 137):
[0304] Step 1) 2-(2-(Trifluoromethyl)phenyl)benzo[d] Preparation of azole-4-carboxylic acid methyl ester (6):
[0305]
[0306] Methyl 2-amino-3-hydroxybenzoate (4; 0.5 g, 3 mmol) and 2-(trifluoromethyl)benzoic acid (5; 0.57 g, 3 mmol) were dissolved in 20 mL of PPA. The reaction mixture was stirred at 140°C for 3 hours. The reaction mixture was cooled to approximately 100°C and carefully diluted with 50 mL of water. The resulting solid was collected by filtration and dried. Purification by chromatography afforded 2-(2-(trifluoromethyl)phenyl)benzo[d] Methyl azole-4-carboxylate 6 (0.3 g, 31%). Calculated MS(ESI): C 16 h 10 f 3 NO 3 : 321.04; measured value: 322 [M+H].
[0307] The other compounds shown in Table 1 were prepared according to the general procedure shown in this procedure using the appropriate carboxylic acid.
[0308] Step ...
Embodiment 3
[0315] Example 3: N-(thiazol-2-yl)-2-(3-(trifluoromethyl)phenyl)benzo[d] Synthesis of azole-7-carboxamide (compound 107):
[0316] Step 1) Preparation of 2-hydroxyl-3-(3-(trifluoromethyl)benzamido)methyl benzoate (11):
[0317]
[0318] Methyl 2-hydroxy-3-nitrobenzoate (9; 1.00 g) was dissolved in 100 mL of a 2:1 THF / MeOH solution. After adding 10% Pd / C (100 mg), the reaction mixture was stirred at room temperature under 1 atm of hydrogen for 18 hours. The reaction mixture was filtered through a plug of Celite and the filtrate was concentrated under reduced pressure to afford crude methyl 3-amino-2-hydroxybenzoate in essentially quantitative yield.
[0319] For the subsequent step, methyl 3-amino-2-hydroxybenzoate 10 (850 mg, 5.1 mmol) was dissolved with 3-trifluoromethylbenzoyl chloride (52; 0.75 mL, 5.1 mmol) at 10 °C In 15mL of pyridine. The resulting reaction mixture was stirred at room temperature for 18 hours, then diluted with 250 mL of EtOAc. The organic layer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com