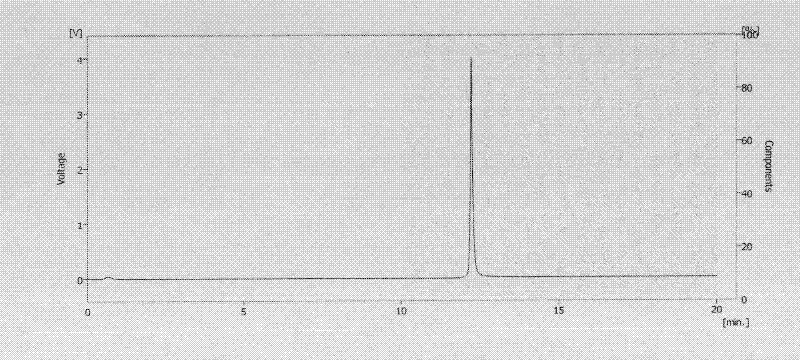Affinity chromatographic packing for separating and purifying monoclonal antibody and antibodyglobulin and preparation method thereof
A monoclonal antibody and chromatographic filler technology, applied in the field of biological separation and purification, can solve the problems of limiting affinity chromatographic separation and purification efficiency, and achieve the effect of increasing specific surface area and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of affinity chromatography filler and affinity chromatography column with polymer polystyrene as solid phase carrier and protein A as ligand:
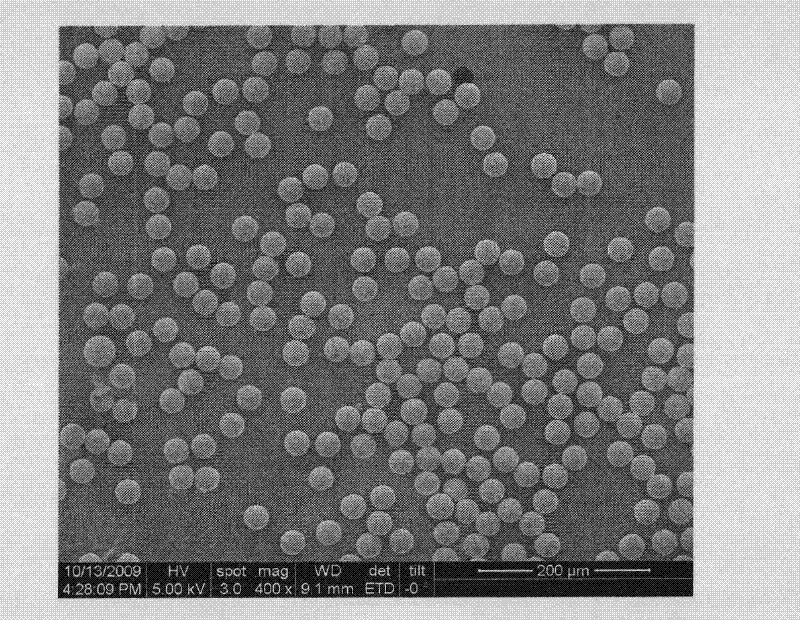
[0044] 1. Choose polystyrene (PS-DVB) porous polymer with uniform particle size and high cross-linking degree as the solid phase carrier. The particle size of the microsphere is about 30 μm, and the pore size of one part is 10-130nm, and the other part is 130-3000nm. It has good mechanical strength and chemical stability, and the surface is coated to enrich the primary alcohol functional group. The film covers the surface of all microspheres, will not be washed off by any organic solvent, and is guaranteed to be used within the pH range of 1-14. Take 10 g of the microspheres and place them in a 100 mL triangular ground-mouth round-bottom flask, which is equipped with a mechanical stirrer in the middle, a water condenser on one side, and a 50 mL dropping funnel on the other side. Mix 60mL of 3mol / L KOH solution and 1...
Embodiment 2
[0050] Isolation of human gamma globulin:
[0051] 1. Chromatographic retention behavior of human gamma globulin on blank column and affinity chromatography column
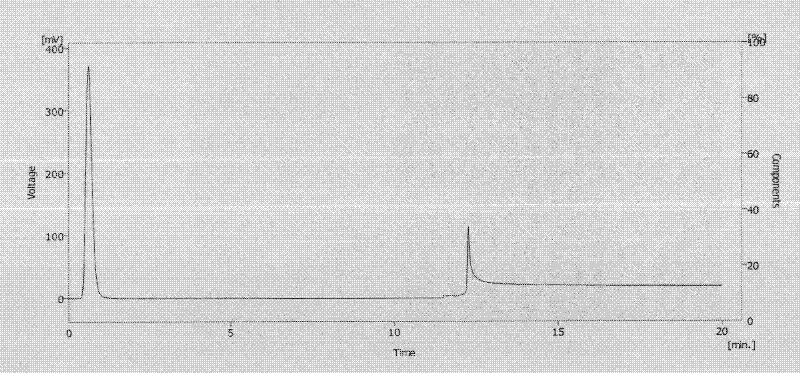
[0052] Connect the affinity chromatographic column or blank column to the high performance liquid chromatography, and the sample is human γ-globulin (Humanγ-Globulin). Mobile phase A was 20mM potassium phosphate buffer (pH 7.5) + 150mM NaCl, and mobile phase B was 0.1M glycine hydrochloride (pH 2.5). Dissolve human gamma globulin in mobile phase A to make a 1.0mg / mL gamma globulin solution, flow rate: 1mL / min; gradient conditions: 0-10min solution A, 10-20min solution B; 20-30min solution A, The injection volume is 20 μL, and the detection wavelength is 254 nm. like Figure 2A , indicating that human gamma globulin is not retained on the blank column, as Figure 2B As shown, it shows that human γ globulin can be retained on the affinity chromatography column and is eluted when the mobile phase is changed to B ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com