Production method of food-grade stabilized chlorine dioxide disinfectant
A production method and chlorine dioxide technology, which are applied in the directions of chlorine oxide, disinfectants, botanical equipment and methods, etc., can solve the problems of high cost, large amount of water vapor consumption, many by-products, etc. Side reactions, the effect of improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
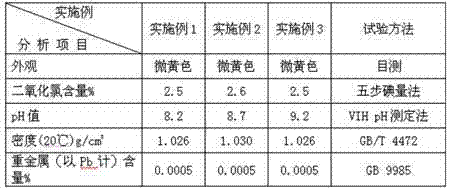
Examples
Embodiment 1
[0040] (1) Methanol, polonium chloride, potassium chloride, acetic acid and sulfuric acid aqueous solution with a mass concentration of 50% were prepared into an acidic reaction solution, and the prepared mass ratio was: 50:0.4:0.4:2.5:1200;
[0041] (2) Continuously drop the acidic reaction solution prepared in step (1) into the saturated sodium chlorate aqueous solution in the reactor, control the pressure of the entire reaction system at 40mmHg, and the temperature of the reaction solution at 60°C;
[0042] (3) The chlorine dioxide gas produced in the step (2) is absorbed in two stages by the absorption liquid in the absorption tower to obtain a stable chlorine dioxide disinfectant; the absorption liquid is composed of water, solid caustic soda (i.e. Sodium hydroxide), solid borax and hydrogen peroxide, their mass ratio is 1:0.06:0.05:0.1.
Embodiment 2
[0044] (1) Methanol, polonium chloride, potassium chloride, acetic acid and sulfuric acid aqueous solution with a mass concentration of 50% are prepared into an acidic reaction solution, and the prepared mass ratio is: 50:0.5:0.5:2.5:1000;
[0045] (2) Continuously add the acidic reaction solution prepared in step (1) dropwise into the saturated sodium chlorate aqueous solution in the reactor, control the pressure of the entire reaction system to 50mmHg, and the temperature of the reaction solution to 70°C;
[0046] (3) The chlorine dioxide gas produced in step (2) is absorbed in two stages by the absorption liquid in the absorption tower to obtain a stable chlorine dioxide disinfectant; the absorption liquid consists of water, solid caustic soda, solid Composed of borax and hydrogen peroxide, their mass ratio is 1:0.06:0.05:0.1.
Embodiment 3
[0048](1) Methanol, polonium chloride, potassium chloride, acetic acid and sulfuric acid aqueous solution with a mass concentration of 50% were prepared into an acidic reaction solution, and the prepared mass ratio was: 50:0.3:0.3:2.5:800;
[0049] (2) Continuously drop the acidic reaction solution prepared in step (1) into the saturated sodium chlorate aqueous solution in the reactor, control the pressure of the entire reaction system to 40mmHg, and the temperature of the reaction solution to 80°C;
[0050] (3) The chlorine dioxide gas produced in the step (2) is absorbed in two stages by the absorption liquid in the absorption tower to obtain a stable chlorine dioxide disinfectant; the absorption liquid is composed of water, solid caustic soda, solid Composed of borax and hydrogen peroxide, their mass ratio is 1:0.06:0.05:0.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com