Preparation method of asymmetric type bi-fluoro sulfimide potassium
A bisfluorosulfonimide potassium, asymmetric technology, applied in the field of preparation of asymmetric bisfluorosulfonyl imide potassium, can solve the problems of high toxicity and high equipment requirements, and achieve reasonable cost and high product purity , purification of simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
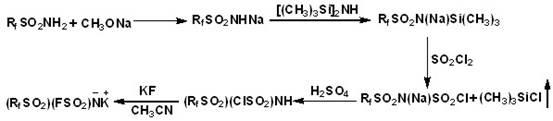
[0024] 1) In a 250mL three-necked flask, add 7.48g (25mmol) perfluorobutylsulfonamide and 1.19g (22mmol) sodium methoxide, 10mL methanol, and add 100mL anhydrous ether to dissolve the solid, heat to reflux for 3 hrs and filter, the filtrate After distillation under reduced pressure, the residue was washed with 30 mL of anhydrous ether to remove excess perfluorobutanesulfonamide, and dried in a vacuum to obtain sodium perfluorobutanesulfonamide, weighing 6.58 g, with a conversion rate of 93%;
[0025] 2) Under the protection of nitrogen, in a 250mL three-necked flask, 5g (15.6mmol) perfluorobutylsulfonamide sodium was dissolved in 100mL anhydrous acetonitrile at room temperature, and 25.1g (156mmol) hexamethyldi Silamine (HMDS) was gradually dropped into the above solution, and heated to reflux at 110 °C for 12 hr; the solvent and excess HMDS were removed under reduced pressure to obtain (C 4 f 9 SO 2 )N(Na)Si(CH 3 ) 3 , which is easy to deliquescence and difficult to obtai...
Embodiment 2
[0030] 1) In a 250mL three-necked flask, add 7.48g (25mmol) perfluorobutanesulfonamide and 1.19g (22mmol) sodium methoxide, 10mL methanol, and add 100mL anhydrous ether to dissolve the solid, heat to reflux for 5 hr and filter, the filtrate Distilled under reduced pressure, the residue was washed with 30 mL of anhydrous ether to remove excess perfluorobutanesulfonamide, and dried in vacuum to obtain sodium perfluorobutanesulfonamide with a conversion rate of 94.5%;
[0031] 2) Under nitrogen protection, in a 250mL three-necked flask, dissolve 5g (15.6mmol) sodium perfluorobutanesulfonamide in 100mL anhydrous acetonitrile at room temperature, and gradually drop 37.7g (234mmol) HMDS just distilled into In the above solution, heat and reflux at 110 ° C for 24 hr; remove the solvent and excess HMDS under reduced pressure to obtain (C 4 f 9 SO 2 )N(Na)Si(CH 3 ) 3 , which is easy to deliquescence and difficult to obtain conversion rate;
[0032] 3) Under the protection of nitro...
Embodiment 3
[0036] 1) In a 250mL three-necked flask, add 20g (40mmol) perfluorooctane sulfonamide and 1.74g (32mmol) sodium methoxide, 20mL methanol, and add 150mL anhydrous ether to dissolve the solid, heat and reflux for 4 hrs and filter, and the filtrate is passed through Distilled under reduced pressure, the residue was washed with 50 mL of anhydrous ether to remove unreacted perfluorooctane sulfonamide, and dried in vacuo to obtain sodium perfluorobutane sulfonamide with a conversion rate of 89%;
[0037] 2) Under the protection of nitrogen, in a 250mL three-necked flask, dissolve 8g (15.4mmol) sodium peroctylsulfonamide in 100mL of anhydrous acetonitrile at room temperature, and gradually drop 24.9g (154mmol) of HMDS just distilled into the above solution, heated to reflux for 36 hr; the solvent and excess HMDS were removed under reduced pressure to obtain (C 4 f 9 SO 2 )N(Na)Si(CH 3 ) 3 , which is easy to deliquescence and difficult to obtain conversion rate;
[0038] 3) Under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com