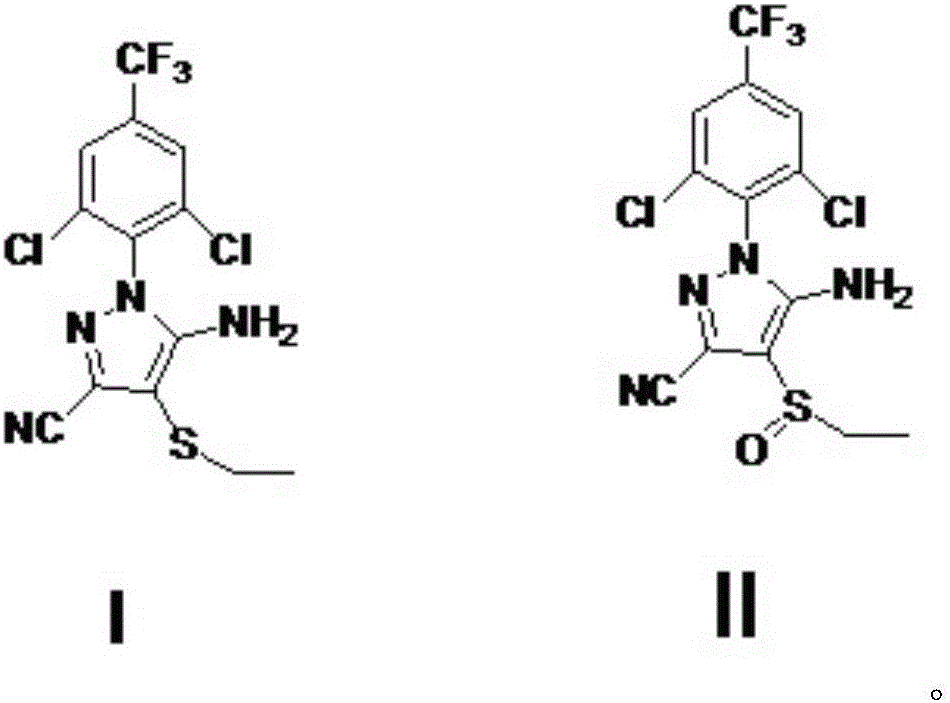
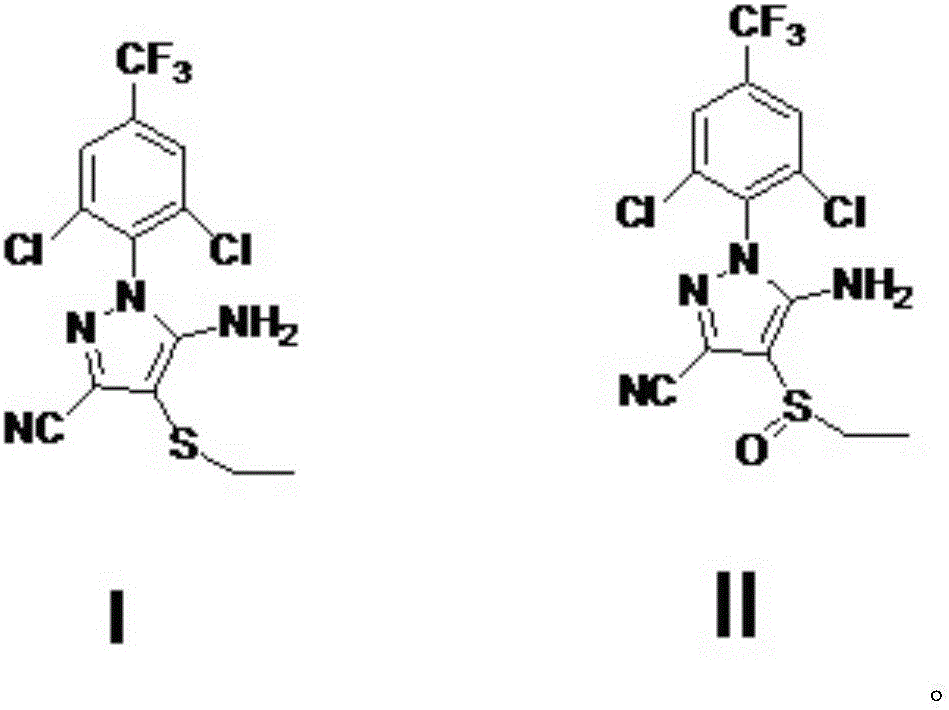
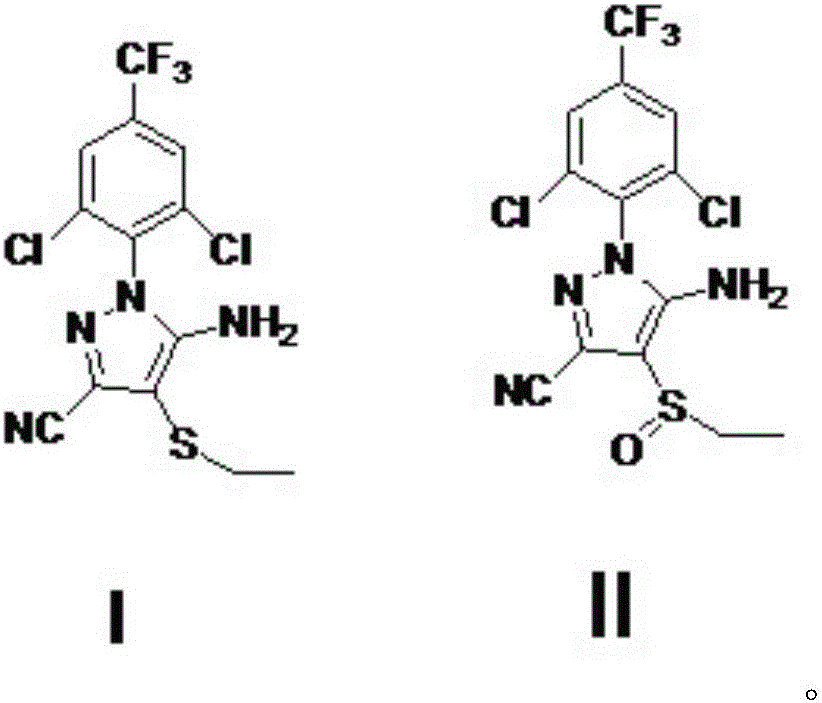
A process for preparing ethiprole by oxidation method
An ethiprole, oxidation technology, applied in the direction of organic chemistry, etc., can solve the problems of high toxicity of raw materials, high pollution, complicated separation and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] With 10.287kg (27mol) acetonitrile oxidation precursor, 107.26kg (1083mol) dichloroethane is added in 100L reactor, add acetic acid 4.212kg (70.2mol) and 35% hydrogen peroxide 6.8175kg (70.2mol), 30 ℃ of stirring about 10 hours. Sampling and tracking, after the reaction is completed, slowly add 3.285kg (31.6mol) of sodium sulfite, the exotherm is obvious, and the temperature is controlled within 55°C. Add sodium hydroxide solution to adjust pH to 6-7, then heat up and reflux (about 80 ℃) and then cool to below 10 ℃, crystallize, suction filtration to obtain 10.50kg of crude solid, purity 98.7%, crude product yield 96%, containing The amount of peroxide impurity is 0.5%, and after one recrystallization, a fine product with a peroxide impurity less than 0.3% is obtained, and the yield of the fine product is 93%.
[0037] Purity of synthetic ethiranil: 99.4% (HPLC).
Embodiment 2
[0039] With 10.287kg (27mol) ethiprole oxidation precursor, 107.26kg (1083mol) dichloroethane layer is added in 100L reactor, add acetic acid 1.62kg (27mol) and 35% hydrogen peroxide 6.8175kg (70.2mol), 30 ℃ of stirring About 20 hours. Sampling and tracking, after the reaction is completed, slowly add 3.285kg (31.6mol) of sodium sulfite, the exotherm is obvious, and the temperature is controlled within 55°C. Add sodium hydroxide solution to adjust pH to 6-7, then heat up and reflux (about 80 ° C) and then cool to below 10 ° C, crystallize, and suction filtration to obtain 10.41kg of crude solid, purity 98.3%, crude product yield 95%, containing The amount of peroxide impurity is 0.6%. After one recrystallization, a fine product with a peroxide impurity less than 0.3% is obtained, and the yield of the fine product is 92%.
[0040] Purity of synthetic ethiranil: 99.3% (HPLC).
Embodiment 3
[0042] With 10.287kg (27mol) acetonitrile oxidation precursor, 107.26kg (1083mol) dichloroethane layer is added in 100L reactor, add acetic acid 4.212kg (70.2mol) and 35% hydrogen peroxide 6.8175kg (70.2mol), 30 ℃ Stir for about 18 hours. Sampling and tracking, after the reaction is completed, slowly add 3.285kg (31.6mol) of sodium sulfite, the exotherm is obvious, and the temperature is controlled within 55°C. Add sodium hydroxide solution to adjust pH to 6-7, then heat up and reflux (about 80 ° C) and then cool to below 10 ° C, crystallize, and suction filtration to obtain 10.40kg of crude solid, purity 97.8%, crude product yield 95%, containing The amount of peroxide impurity is 0.8%, and after recrystallization twice, a fine product with a peroxide impurity less than 0.3% is obtained, and the yield of the fine product is 91%.
[0043] Purity of synthetic ethiranil: 99.1% (HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com