Method for preparing propylene and aromatic hydrocarbon by virtue of conversion of methanol
A technology for producing propylene and methanol from methanol, which is applied in the production of hydrocarbons from oxygen-containing organic compounds, organic chemistry, ethylene production, etc., can solve problems such as inability to co-produce aromatics, and achieve the effect of improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
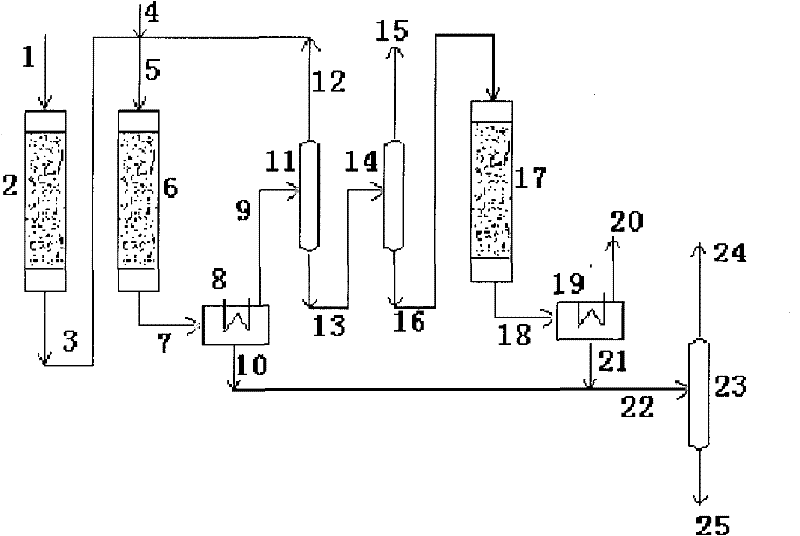
[0021] according to figure 1 In the process flow shown, the raw material methanol enters the pre-reactor 2 through the pipeline 1, and the reaction conditions are controlled: the reaction temperature is 200°C, the reaction pressure is 0.1MPa, and the methanol weight space velocity is 1.0h -1 , at this moment, the conversion rate of methanol is 80%, and the selectivity of dimethyl ether is 100%. The reaction product dimethyl ether and unreacted methanol are mixed in pipeline 5 with water vapor coming from pipeline 4 through pipeline 3, and enter methanol-to-propylene reactor 6, wherein the catalyst composition is 88% HZSM-5, 0.02% P, and the rest As a binder, the control reaction conditions are: reaction temperature 420°C, reaction pressure 0.02MPa, H 2 O / CH 3 OH=1.50 / 1 (mass), methanol weight space velocity 2.0h -1 At this time, the conversion rate of methanol and dimethyl ether is 100%, the selectivity of propylene is 33%, the selectivity of ethylene is 13.2%, and the sele...
Embodiment 2
[0023]Process flow is with embodiment 1. The pre-reaction conditions are: reaction temperature 220°C, reaction pressure 0.05MPa, methanol weight space velocity 2.0h -1 At this time, the conversion rate of methanol is 82%, and the selectivity of dimethyl ether is 100%. The catalyst composition of methanol to propylene reaction is 80% HZSM-5, 1.0% P, and the rest is binder, the reaction temperature is 450°C, the reaction pressure is 0.05MPa, H 2 O / CH 3 OH=1 (mass), methanol weight space velocity 3.0h -1 At this time, the conversion rate of methanol and dimethyl ether is 100%, the selectivity of propylene is 34.5%, the selectivity of ethylene is 12%, and the selectivity of C4C5 is 36.5%. The rest except a small amount of methane and ethane are mainly liquid phase products. The composition of aromatization reaction catalyst is 70% HZSM-5, 0.2% Ga, the rest is binder, the reaction temperature is 530°C, the reaction pressure is 0.5MPa, and the weight space velocity is 3.0h -1 , ...
Embodiment 3
[0025] Process flow is with embodiment 1. The pre-reaction conditions are: reaction temperature 250°C, reaction pressure 0.08MPa, methanol weight space velocity 3.0h -1 At this time, the conversion rate of methanol is 84%, and the selectivity of dimethyl ether is 100%. The catalyst composition for the reaction of methanol to propylene is 75% HZSM-5, no P is added, the rest is binder, the reaction temperature is 480°C, the reaction pressure is 0.08MPa, H 2 O / CH 3 OH=1.5 / 1 (mass), methanol weight space velocity 4.0h -1 At this time, the conversion rate of methanol and dimethyl ether is 100%, the selectivity of propylene is 36.8%, the selectivity of ethylene is 11.2%, and the selectivity of C4C5 is 36%. The rest except a small amount of methane and ethane are mainly liquid phase products. The composition of aromatization reaction catalyst is 75% HZSM-5, 1.0% Ga, and the rest is binder, the reaction temperature is 550°C, the reaction pressure is 0.8MPa, and the weight space vel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com