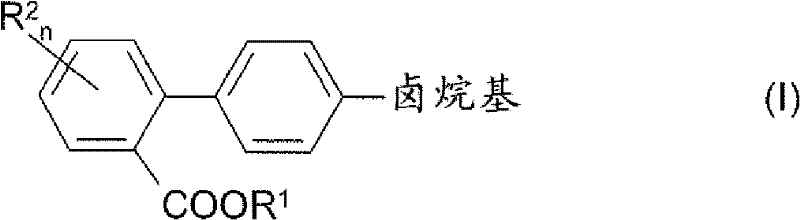
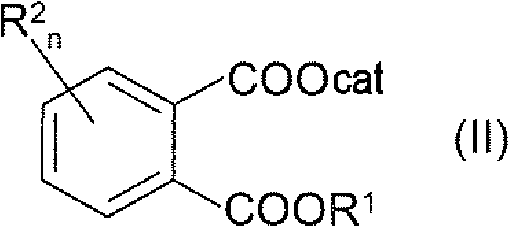
Process for preparing 4'-halogenalkyl-biphenyl-2-carboxylic acids
An alkyl and aralkyl technology, applied in the fields of chemicals for biological control, carboxylate preparation, chemical instruments and methods, etc., can solve the problems of safety, unsuitable cost, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0105] 1. Preparation of monoisopropyl phthalate
[0106]Suspend phthalic anhydride (14.8 g, 100 mmol) in a 100 ml round bottom flask with reflux condenser i PrOH (isopropanol) (50ml) and heated at the boil for 6 hours. After cooling the clear solution to room temperature, it was concentrated by evaporation and the remaining colorless oily residue was dried in vacuo, during which time it slowly crystallized out and the product was obtained as a colorless solid ( 20.8 g, 99%). 1 H-NMR (600MHz, CDCl 3 )δ=12.46(s, 1H), 7.85(d, 3 J=7.6Hz, 1H), 7.64(d, 3 J=7.6Hz, 1H), 7.54(t, 3 J=7.6Hz, 1H), 7.49(t, 3 J=7.6Hz, 1H), 5.24(h, 3 J=6.2Hz, 1H), 1.32(d, 3 J = 6.7 Hz, 6H) ppm. 13 C-NMR (151MHz, CDCl 3 ) δ = 172.6, 167.5, 133.7, 132.0, 130.4, 129.6, 129.5, 128.5, 69.5, 21.3 ppm.
[0107] 2. Preparation of Potassium Monoisopropyl Phthalate
[0108] Monoisopropyl phthalate (10.4 g, 50.0 mmol) was dissolved in 2-propanol (20 ml) in a 250 ml round bottom flask. A solution of potass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com