Synthesis method of sodium glycididazole
A kind of technology of glycidazole sodium and synthesis method, applied in the field of medicinal chemistry, can solve the problems such as the possibility of ammonium triazole ester that cannot be ruled out and the total yield is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
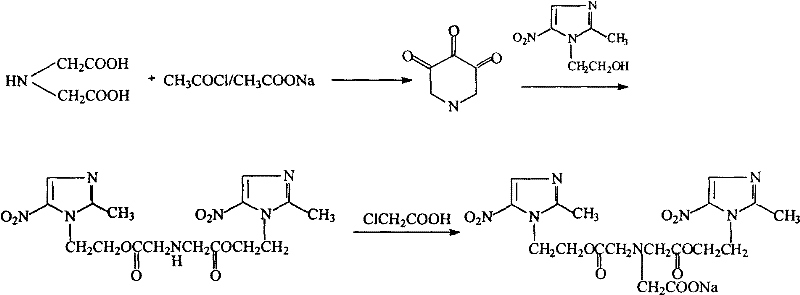
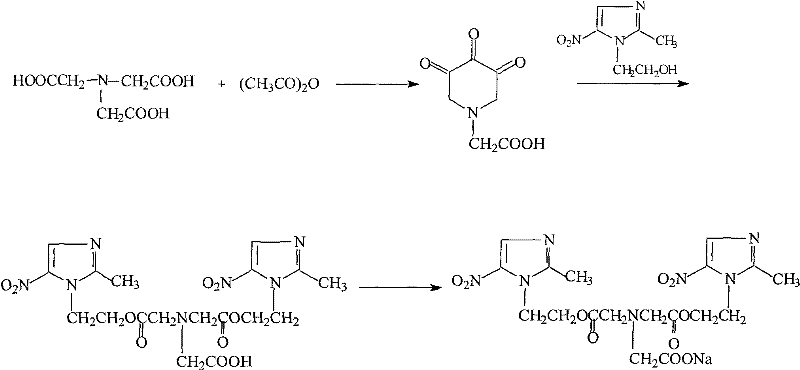
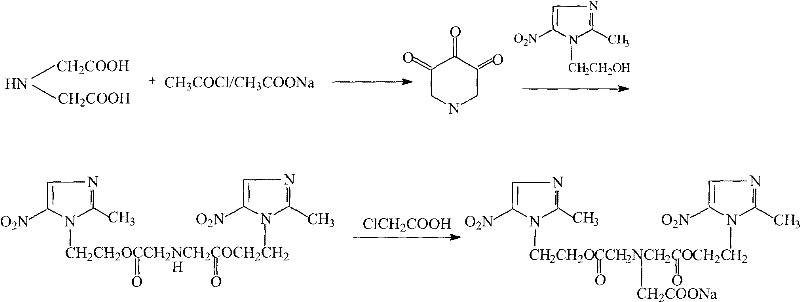
[0035] Embodiment 1, the preparation of iminoacetic anhydride-esterification
[0036] (1) 266.2g (2mol) iminodiacetic acid, 220ml (2.9mol) acetyl chloride, 238g (2.9mol) anhydrous sodium acetate and 1600ml DMF were added successively in the three-necked flask of 3000ml, stirred for half an hour, until the stirring was roughly uniform ;
[0037] (2) Continue to stir and heat the oil bath to gradually increase the internal temperature of the system from 30°C to 50°C;
[0038] (3) React at 50°C for 5-6h;
[0039] (4) stop stirring and heating, add 456g metronidazole;
[0040] (5) stirring, heating in an oil bath, raising the internal temperature of the system to 40°C--45°C, and reacting for 2h;
[0041] (6) stop stirring and heating, pour the reaction solution into 2000ml of water, adjust the pH value to 3-4, freeze at -10°C, let stand overnight, and separate out the first batch of imidobisazole ester;
[0042] (7) The filtrate was diluted with 2000ml of water, then frozen at...
Embodiment 2
[0043] Embodiment 2, the preparation-condensation, salify of sodium glycidazole
[0044] (1) in the three-necked flask of 2000ml, successively add 438.2g (1.0mol) imidobisazole ester, 100ml (1.1mol) chloroacetic acid, add 1000ml THF, 67g (1.1mol) sodium acetate;
[0045] (2) Continue stirring and heat the oil bath to raise the system from 30°C to 70-80°C;
[0046] (3) stirring reaction under 70-80 ℃ condition for 4 hours, cooling to room temperature;
[0047] (4) under ice bath condition, slowly drip the ethanol solution of 49ml 5.8% NaOH into the system (200ml ethanol dissolves 11.6g NaOH);
[0048] (5) complete dropwise addition, remove ice bath, stir at room temperature for 24 hours, a small amount of white solid has been separated out;
[0049] (6) Stop stirring, let the solution stand at room temperature for 24 hours, a large amount of solids are precipitated, and 451.6 g of glycidazole sodium salt is obtained by suction filtration, with a yield of 80.5%.
Embodiment 3
[0050] Embodiment three, the refining of glycidazole sodium
[0051] Put 580g of sodium glycidazole crude product into a 2000ml reaction flask, add 800ml of purified water, 200g of 95% ethanol, heat to 40-50°C under stirring, stir to dissolve completely, add activated carbon and an appropriate amount of sodium ETDA with stirring, keep warm for 30 minutes, and take Hot filtration, the filtrate was cooled to 5-10°C, anhydrous ethanol was added under stirring, cooled to 0-5°C, crystals were precipitated, and 464 g of sodium glycobisazole sodium (containing 3 crystal waters) was dried as a white crystalline powder, yield 80 -86%. Glycibisazole sodium salt content (HPLC based on anhydrous): 99.6%, infrared and mass spectrometry are consistent with pharmaceutical standard samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com