Fusion protein TAT (transactivator of transcription)-OCT4 (octamer-binding transcription factor 4), and coding gene and application thereof
A protein and gene technology, applied in the fusion protein TAT-OCT4 and its encoding gene and application field, can solve the problem that the function of a single inducer is rarely studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, TAT transmembrane peptide nuclear transcription factor construction
[0040] The total RNA of PA-1 cells (purchased from ATCC) was extracted using TRIZOL kit (purchased from Invitrogen), digested with DNase I (purchased from Invitrogen), and the above PA-1 cells were digested with reverse transcriptase Super Transcript III (Invitrogen). The total RNA of 1 cell was reverse-transcribed into cDNA, and using the cDNA as a template, the upstream primer 5'-GCGGAGCTCATGGCGGGACACCTGG-3' and the downstream primer 5'-ATAGCGGCCGCTTATCAGTTTGAATGCA-3' of OCT4 were designed respectively, and the upstream primer of NANOG 5'-GAGCTCATGAGTGTGGATCCAGCTT- 3' and downstream primer 5'-GCGGCCGCTCACACGTCTTCAGGTT-3', with Pfx DNA polymerase (purchased from Invitrogen) was used for PCR reaction.
[0041] The above PCR reaction product was recovered and detected by 1.0% agarose gel electrophoresis. As a result, a 1090bp OCT4 amplification product and a 918bp NANOG amplification pr...
Embodiment 2
[0045] Embodiment 2, expression and purification of TAT transmembrane peptide nuclear transcription factor
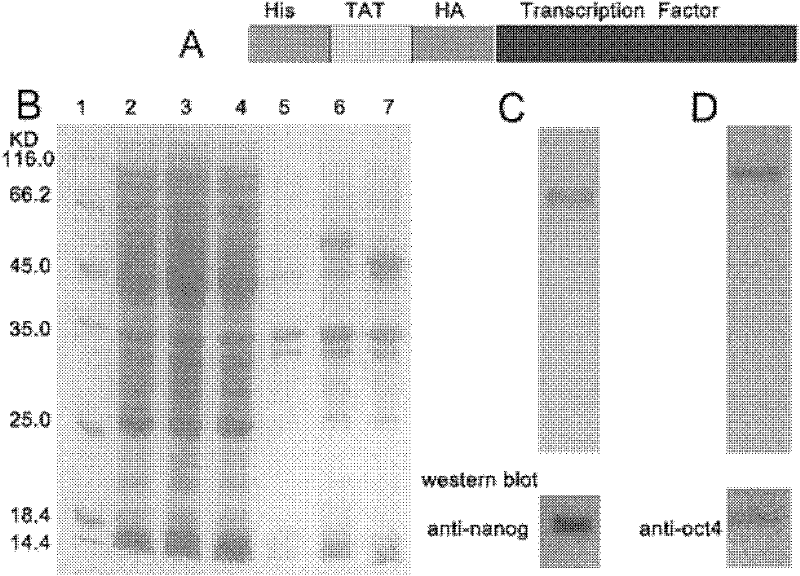
[0046] The expression vectors PET-TAT-OCT4, PET-TAT-NANOG and the control empty vector pET28a were transformed into the BL21 Rostta-Gami (purchased from Invitrogen) strain of Escherichia coli E. After induction of expression from Sigma Company) for 5 hours, the bacteria were collected by centrifugation, ultrasonically broken, and the target protein was purified through a nickel column (purchased from GE Company) using the HIS tag, and finally the OCT4 antibody (purchased from Santa Cruze Company) was used to ) and NANOG antibodies were used to identify the target protein by western blot. The results of expression purification and identification are as follows: figure 1 shown. The amino acid sequence of the fusion protein (TAT-OCT4) expressed by the expression vector PET-TAT-OCT4 is shown in sequence 8 in the sequence listing. The 1-21st position of sequence 8 in the ...
Embodiment 3
[0047] Example 3, Cell transfection detection of TAT transmembrane peptide nuclear transcription factor
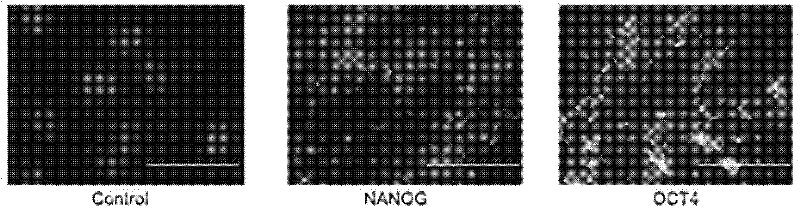
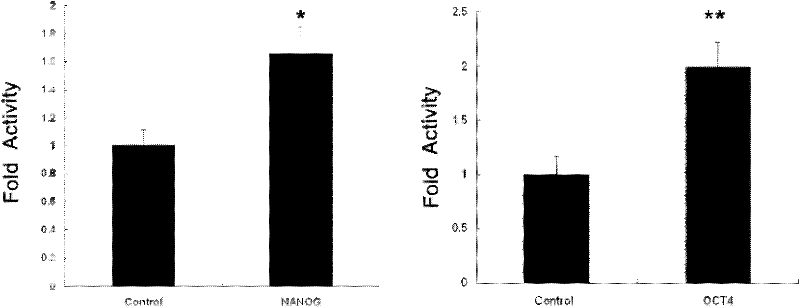
[0048] We added TAT-NANOG and TAT-OCT4 with a final concentration of 0.2M to the culture medium of HAF cells, and after 2 hours, we used the antibody of HA (purchased from Sigma Company) to detect the translocation of TAT fusion protein by immunofluorescence. Membrane efficiency was tested. The results showed that the TAT fusion protein had passed through the cell membrane and entered the cytoplasm, and part of the protein had been localized in the nucleus ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com