Stable exenatide sustained-release microsphere preparation and preparation method thereof
A technology of slow-release microsphere preparation and exenatide, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of poor patient compliance and achieve reasonable composition, The effect of good technical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
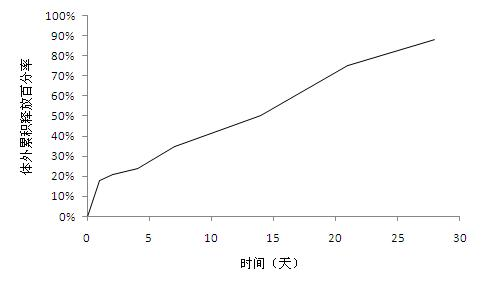
[0031] Weigh 800mg of exenatide and 300mg of gelatin and dissolve it in 1ml of water for injection as the inner water phase; weigh 6g of glycolide-lactide copolymer and dissolve it in 15ml of dichloromethane as the oil phase; add the oil phase to In the inner water phase, use high-speed stirring (23000rpm) for 15 seconds to obtain colostrum and store it in an environment below 20°C; add this colostrum to 6000 ml of 0.5% (W / V) polyethylene under stirring The alcohol solution was used to obtain double emulsion, and the double emulsion was continuously stirred for 3 hours to evaporate dichloromethane, centrifuged, washed and collected to obtain microspheres, the particle size of which was less than 100 microns. The drug loading capacity was 9.8%, and the encapsulation efficiency was 87.0%.
Embodiment 2
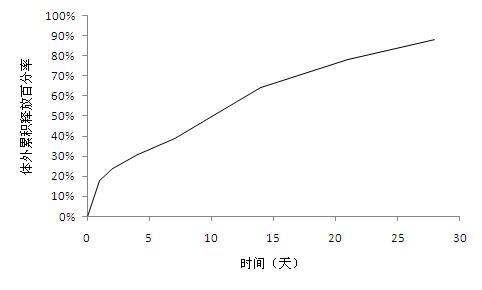
[0033]Weigh 200mg of exenatide, 300mg of carboxymethylcellulose sodium and 100mg of sucrose and dissolve in 1ml of water for injection as the inner aqueous phase; weigh 12.5g of glycolide-lactide copolymer and dissolve in 50ml of dichloromethane As the oil phase; add the oil phase to the inner water phase, and use high-speed stirring (23000rpm) for 15 seconds to obtain colostrum, which is stored in an environment below 20°C; add this colostrum to 6000 ml of 0.5 % (W / V) polyvinyl alcohol solution to obtain double emulsion. The double emulsion was continuously stirred for 3 hours to evaporate dichloromethane, centrifuged and washed to collect microspheres, and the particle size of the microspheres was less than 100 microns. The drug loading is 0.5%, and the encapsulation efficiency is 32.7%.
Embodiment 3
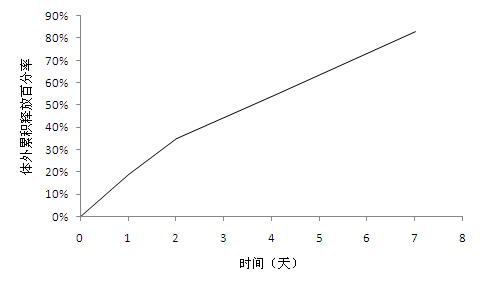
[0035] Weigh 500mg exenatide, 300mg gelatin and 100mg mannitol and dissolve in 2ml water for injection as the inner water phase; weigh 8g glycolide-lactide copolymer and dissolve it in 80ml of dichloromethane as the oil phase; The oil phase was added to the inner water phase, and the ultrasonic cell breaker (Branson S-250D) was used to perform ultrasonication for 30 seconds to obtain colostrum, which was stored in an environment below 20°C; the colostrum was added to 10,000 Milliliter of 1.0% (W / V) polyvinyl alcohol solution was used to obtain double emulsion, and the double emulsion was continuously stirred for 3 hours to evaporate methylene chloride, centrifuged and washed to collect microspheres, the particle size of which was less than 50 microns. The drug loading was 4.2%. The encapsulation rate is 74.7%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com