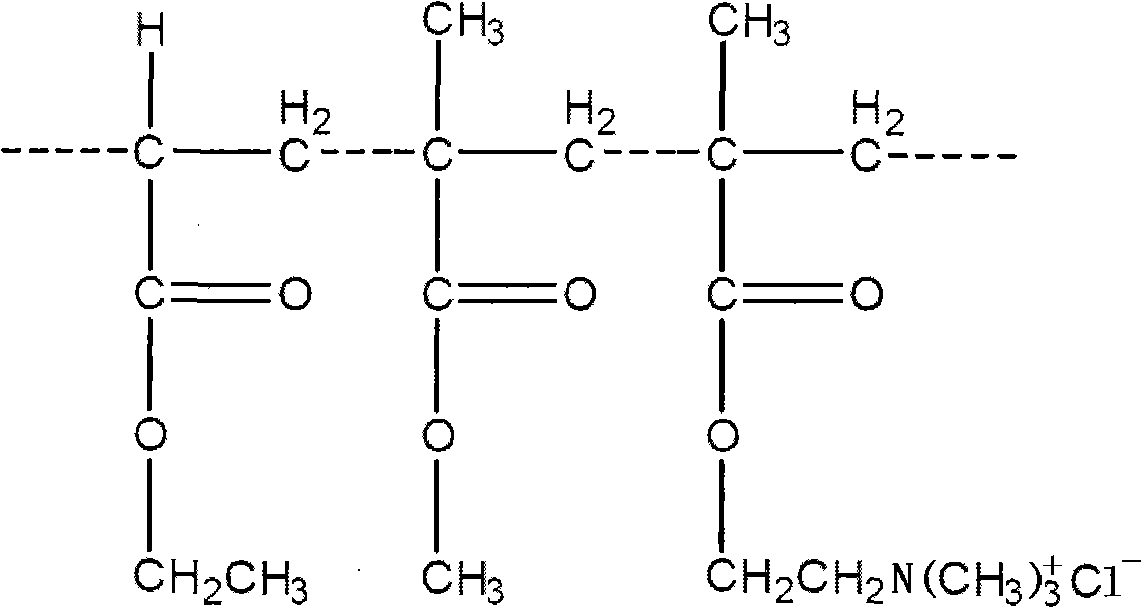
Osmotic pump type controlled release tablets and preparation method thereof
A technology of osmotic pumps and controlled-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, drug delivery, etc., can solve problems such as undiscovered, and achieve the effects of avoiding superposition, precise release control, and strong toughness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
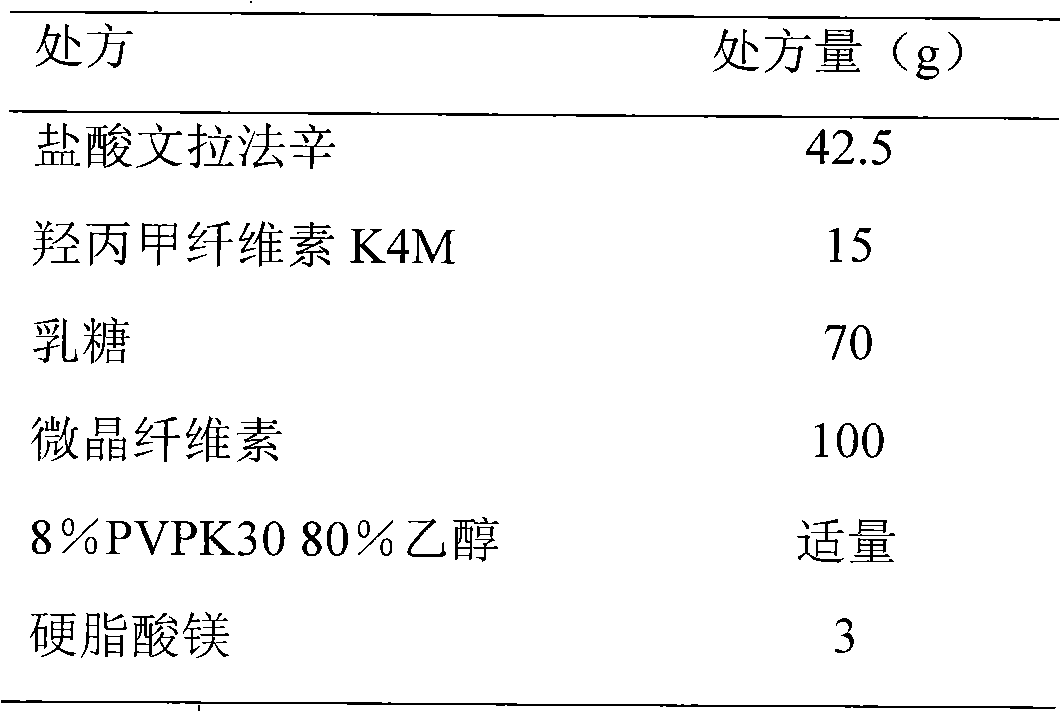
[0059] Example 1 Venlafaxine hydrochloride immediate release + controlled release osmotic pump tablet formulation, preparation method and release characteristics prepared by common semipermeable membrane material
[0060] 1. Prescription
[0061] 1. Tablet core prescription (1000 tablets) (specification 37.5mg)
[0062]
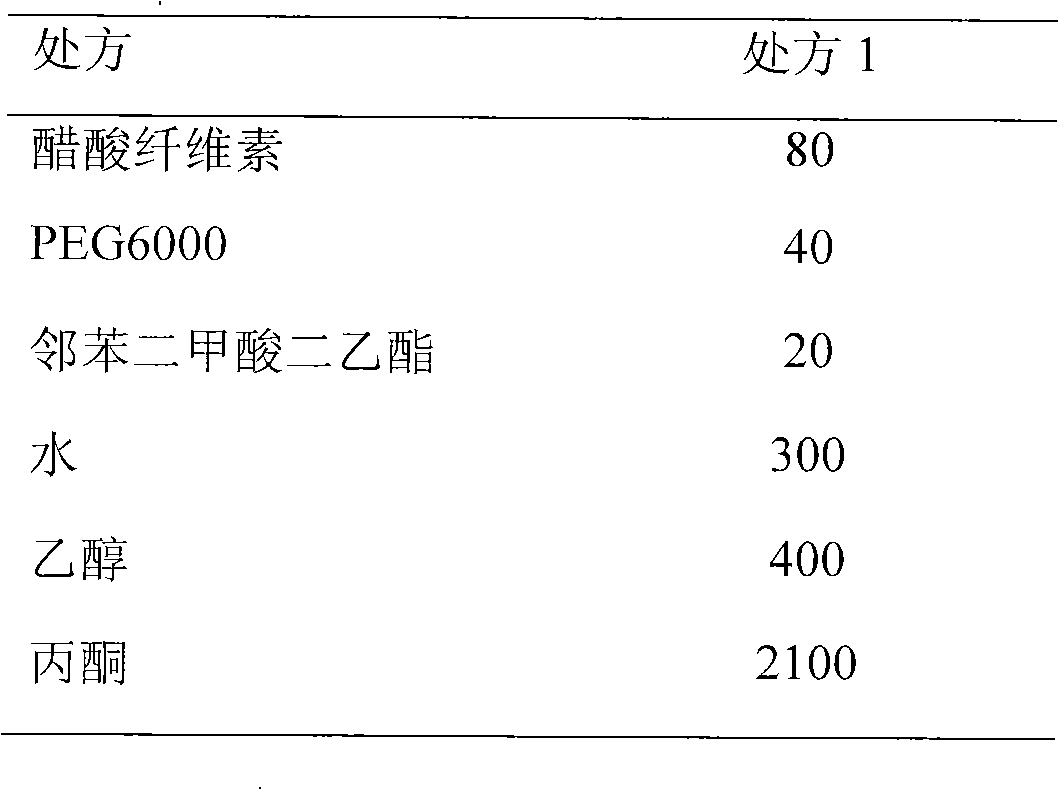
[0063] 2. Semi-permeable membrane prescription (1000 tablets)
[0064]
[0065] 3. Immediate release layer prescription
[0066]
[0067] 2. Preparation process
[0068] 1. Tablet core preparation process
[0069] Weigh venlafaxine hydrochloride, hypromellose K4M, lactose, and microcrystalline cellulose of the prescribed amount and mix them evenly; make soft materials with 8% PVPK30 and 80% ethanol; pass through a 24-mesh sieve for granulation, and dry at 40°C; Sieve with a 24-mesh sieve; add the prescribed amount of magnesium stearate and mix well, calculate the theoretical tablet weight, and punch the tablet with a 9mm circle.
[0070] 2. Prep...
Embodiment 2
[0086] Example 2 Venlafaxine hydrochloride immediate release-controlled release osmotic pump tablet using cellulose acetate-acrylic resin RL as semipermeable film-forming material
[0087] 1. Prescription
[0088] 1. Tablet core prescription (1000 tablets) (specification 37.5mg)
[0089]
[0090] 2. Semi-permeable membrane prescription (1000 tablets)
[0091]
[0092] 3. Immediate release layer prescription
[0093]
[0094] Second, the preparation process:
[0095] 1. The preparation process of the tablet core: pass venlafaxine hydrochloride through a 100-mesh sieve, and pass other auxiliary materials through a 60-mesh sieve for later use; weigh the main drug and other auxiliary materials (except lubricant) according to the prescription ratio; make soft with a wetting agent material; pass through a 24-mesh sieve to granulate, dry at 40°C, add the prescribed amount of lubricant, pass through a 24-mesh sieve for granulation; calculate the theoretical tablet weight, ...
Embodiment 3
[0111] Example 3 Venlafaxine hydrochloride immediate-release-controlled release osmotic pump tablet using cellulose acetate-acrylic resin RL as semipermeable film-forming material
[0112] 1. Prescription
[0113] 1. Tablet core prescription (1000 tablets) (specification 50mg)
[0114]
[0115] 2. Semi-permeable membrane prescription (1000 tablets)
[0116]
[0117] 3. Immediate release layer prescription
[0118]
[0119] 2. Preparation process: the same as in Example 2, wherein when the specifications of the controlled-release part are 50 mg, and the specifications of the immediate-release part are 12.5, 25, 37.5, and 50 mg (calculated as venlafaxine), the weight gain of each immediate-release layer coating is about 21.23 mg, 42.47mg, 63.70mg, 84.94mg.
[0120] 3. Release measurement method and results
[0121] 1, measurement method: with embodiment 2, wherein the sample measurement of specification 50mg controlled release uses 2cm absorption cell, all the other...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com