Amphipathic conjugate anti-tumor nano-drug with function of reversing multidrug resistance of tumors and preparation method and application thereof
A multi-drug resistance and anti-tumor drug technology, applied in the field of anti-tumor drugs, can solve problems such as multi-drug resistance and inflammation of kidneys and other organs, so as to increase drug concentration, reverse multi-drug resistance of tumors, reduce Toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
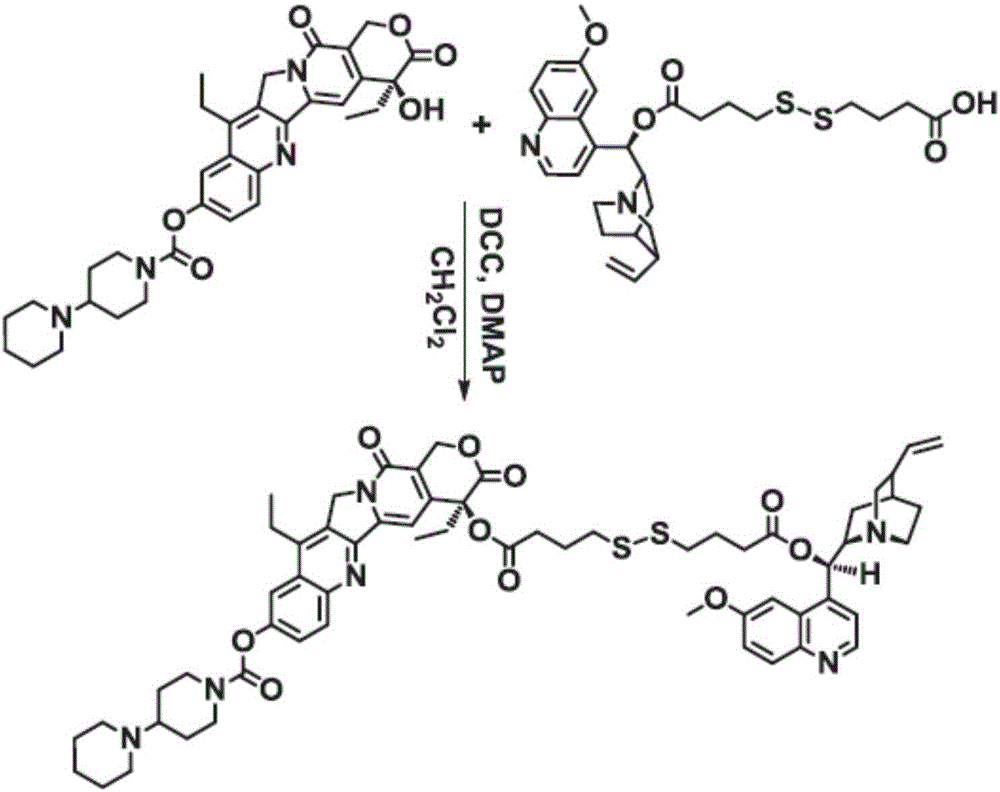
[0039] Embodiment 1: A kind of amphiphilic conjugate Ir-ss-Qu antitumor nano drug prepared by quinine Qu (formula 1) and irinotecan Ir (formula 2) of the present invention
[0040]
[0041] 1. The preparation method of the amphiphilic conjugate Ir-ss-Qu anti-tumor nano-drug specifically comprises the following steps:
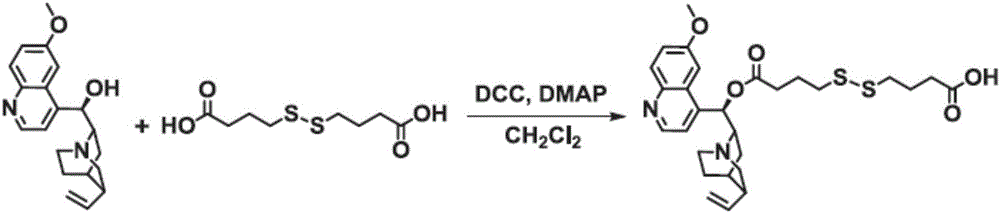
[0042] (1) Synthesis of intermediate Qu-ss-COOH
[0043] Such as figure 1 As shown, at room temperature, respectively, in 250mL dry and clean round bottom flasks, add 4,4'-dithiodibutyric acid (4.77g, 20mmol), dicyclohexylcarbodiimide (2.27g, 11mmol) , 4-methylaminopyridine (1.22g, 10mmol) and 100mL of anhydrous tetrahydrofuran, after stirring and reacting for 30min, then 50mL of tetrahydrofuran solution dissolved with quinine (Qu, 3.24g, 10mmol) was added dropwise to the above reaction system, The reaction was continued for 24h, and after the reaction was finished, the organic solvent was removed by rotary evaporation to obtain the crude product, which was...
Embodiment 2
[0065] Embodiment 2: A kind of amphiphilic conjugate antitumor nano drug prepared by quinine Qu (formula 1) and irinotecan Ir (formula 2) of the present invention
[0066] 1. Another preparation method of the amphiphilic conjugate Ir-cc-Qu anti-tumor nanomedicine, which specifically includes the following steps: Example 2 is the same as Example 1 except for the following steps.
[0067] (1) Synthesis of intermediate Qu-cc-COOH
[0068] Take 100mL reaction flask, add quinine (324mg, 1mmol), succinic anhydride (500mg, 5mmol), DMAP (122mg, 1mmol) and 50mL anhydrous CH 2 Cl 2 , under the protection of nitrogen, react in the dark at 75°C for 48 hours, after the reaction, remove the solvent CH with a rotary evaporator 2 Cl2, then the crude product was purified through a silica gel column (CH 2 Cl 2 :CH 3 OH=20:1, v / v), after collection, the organic solvent was removed by rotary evaporation to obtain a light yellow solid Qu-COOH (237 mg, yield: 51.1%).
[0069] (2) Synthesis of...
Embodiment 3
[0072] Example 3: An amphiphilic conjugate Ir-ss-Qu anti-tumor nano drug prepared by quinine Qu (formula 1) and irinotecan Ir (formula 2) of the present invention
[0073] 1. Another preparation method of the amphiphilic conjugate Ir-ss-Qu anti-tumor nano-medicine, which specifically includes the following steps: In Example 3, except for the following steps, other steps are the same as in Example 1
[0074] (1) Synthesis of intermediate Qu-ss-OH
[0075] Take a 240mL reaction bottle, add quinine (972mg, 3mmol), triphosgene (311.6mg, 1.05mmol), DMAP (1.22mg, 10mmol) and 120mL anhydrous CH 2 Cl 2 , under the protection of nitrogen, after reacting for 1 hour at room temperature in the dark, add 2,2′-dithiodiethanol (2.26 g, 3.85 mmol) in 50 mL of CH 2 Cl 2 Solution, continue to react for 24h, after the end of the reaction, wash 3 times with 1N HCl, then wash with 10% saturated NaHCO 3 Solution was washed 3 times, saturated brine once, deionized water twice, and anhydrous Na ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com