Novel method for oxidative folding of protein
A protein and oxidant technology, applied in chemical instruments and methods, peptide preparation methods, organic chemistry, etc., to reduce production costs, facilitate production operations, and simple ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The statements of the following embodiments are for better understanding of the principle and application of the present invention, but not to limit the application scope of the present invention.
[0031] Materials and Methods:
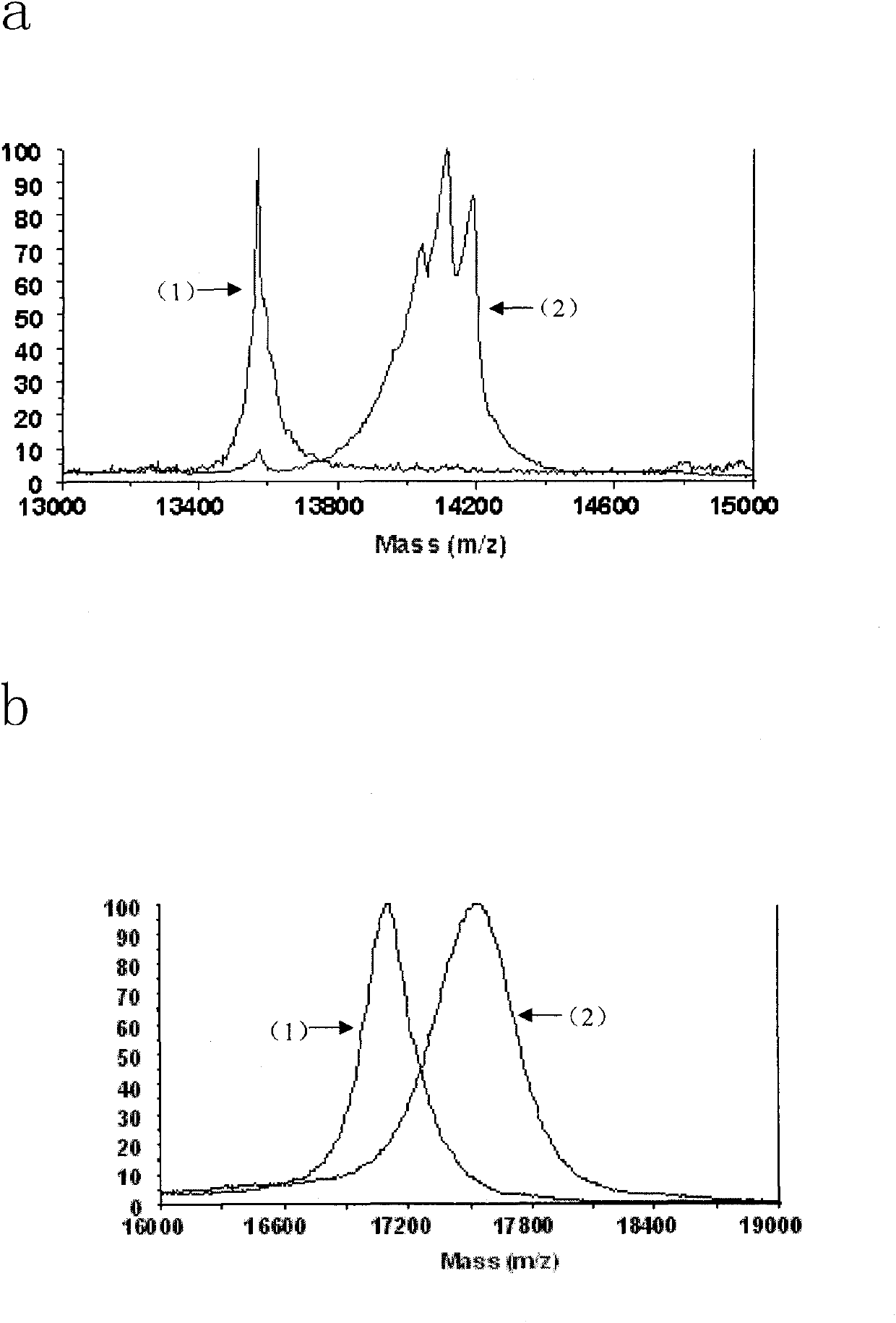
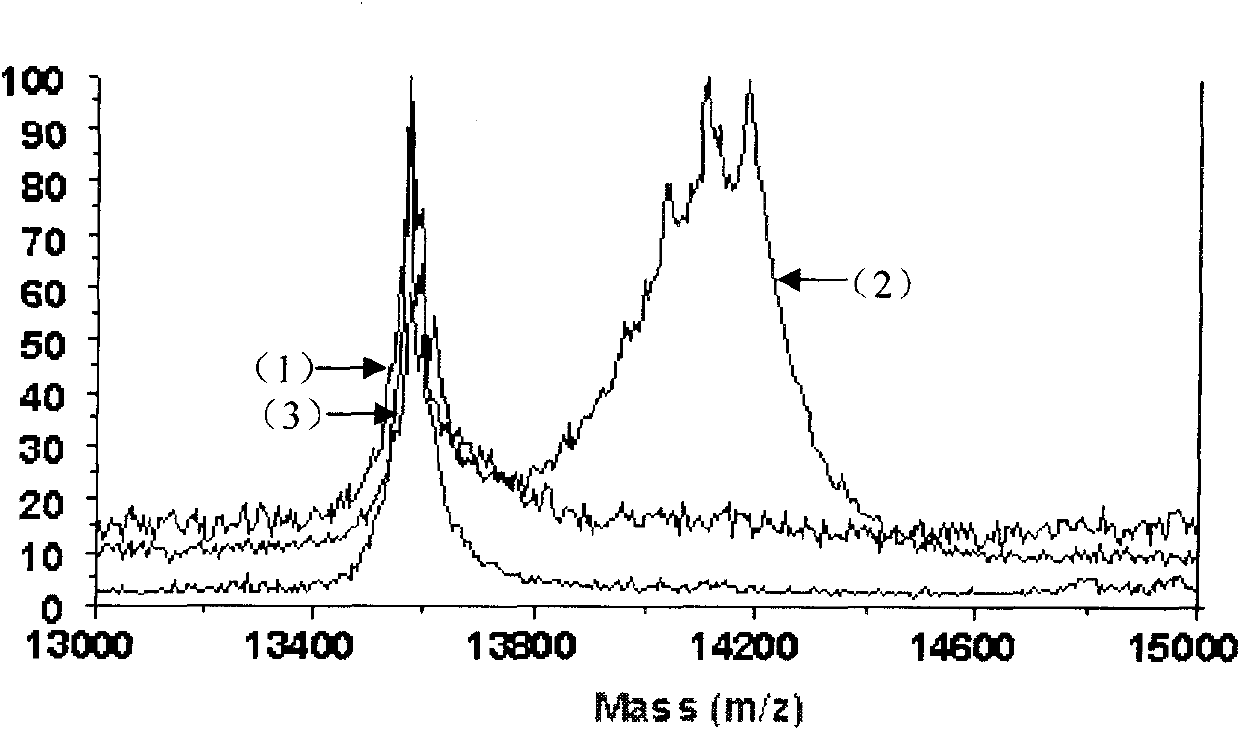
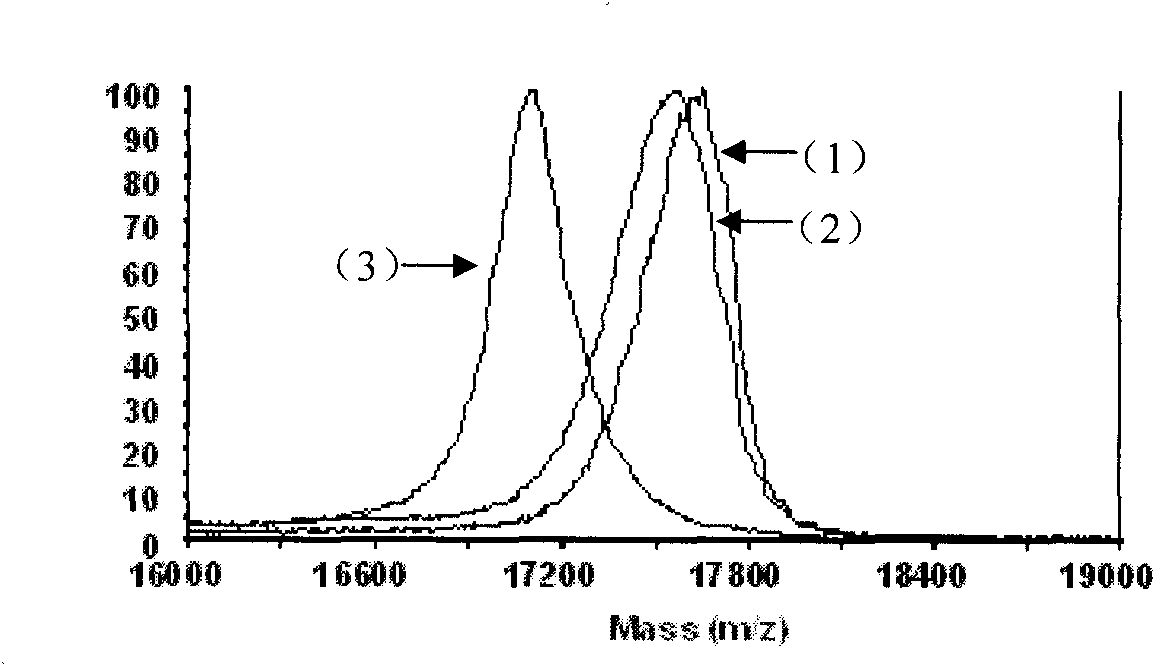
[0032] 1. Protein sample: bovine pancreatic RNase A was purchased from Beijing Qinyuan Huizhi Biotechnology Co., Ltd.; electrophoresis and mass spectrometry analysis conformed to its theoretical molecular weight (13689.33). The cDNA encoding human pancreatic RNase I was amplified from the reverse transcription product of total RNA of human pancreatic cancer cell PANC-1 by PCR reaction. The gene encodes a mature human pancreatic RNase I protein of 128 amino acid residues. Using the gene as a template, primers are designed to amplify gene fragments encoding S peptide (1-15 amino acid sequence) and S protein (21-128 amino acid sequence). A mutant gene encoding S protein C-terminal fusion S peptide was generated by enzyme-cleavage ligation. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com