Method for efficiently producing recombinant proteins in mammary glands by utilizing artificial chromosomes
An artificial chromosome and mammary gland technology, applied in the field of genetic engineering, can solve the impossible and other problems, and achieve the effect of improving the success rate, saving costs and overcoming the position effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Example 1 Construction of expression vector pBAC-hLF-hLZ-Neo hybrid BAC (taking human lysozyme gene and human lactoferrin BAC as examples)
[0035] 1.1 Construction of human lysozyme mammary gland expression vector
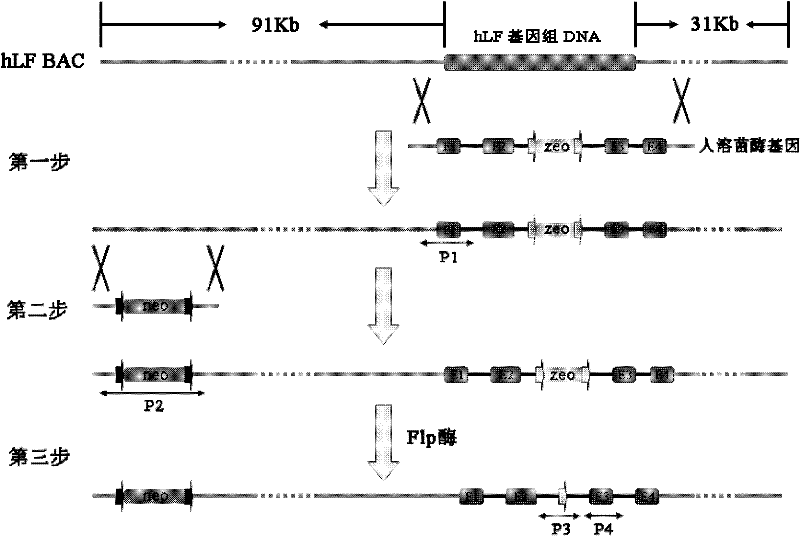
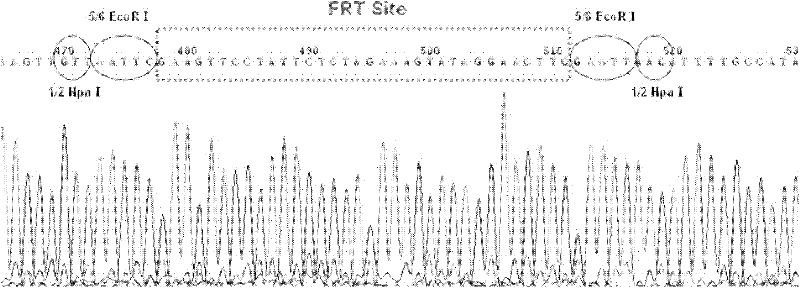
[0036] The construction process of the expression vector pBAC-hLF-hLZ-Neo is divided into three steps, such as figure 1 Shown:
[0037] In the first step, the hLZ genomic DNA completely replaces the target gene on the hLF BAC. Using hLZBAC as a template, a 4.8kb hLZ gene with homology arms was amplified;
[0038] Primer: hLF-hLZ-F (5'-CTA GCTAGC AAAGCCCTGAATAAAGGGGCGCAGGGCAGGCGCAAGTGGCAGAGCCTTCGTTTGCCAAGTCGCCTCCAGACCGCAGACATGAAGGCTCTCATTGTTCTG-3') and hLF-hLZ-R(5'-CTA GCTAGC AGGGGAGGCCAAGGCCCCAACACACCTGGGGAGAAGAGCTGGGGGCAGTGAATGGCTGAGGCTTTCTTGGGGAGCTGGGCCATCTTCTTCGGTTTTACA
[0039] The underline is the NheI restriction site, and the bold font is the homology arm. The PCR product was connected into the pMD19-T vector and sequenced to verify correctne...
Embodiment 2
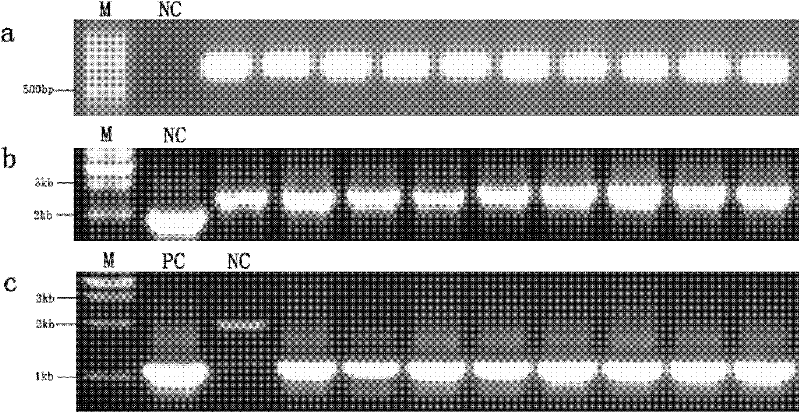
[0055] The establishment and detection of embodiment 2 transgenic mouse model
[0056] 2.1 Obtaining transgenic mice by microinjection
[0057] The production process of transgenic mice is as follows: Figure 12 shown.
[0058] For the preparation process of transgenic mice, please refer to "Experimental Guide for Mouse Embryo Operation" (A. Naji et al., 2004, Science Press). The present invention uses circular BAC to inject mice, which has the same transgenic efficiency as linear BAC.
[0059] Microinjection is used to produce transgenic mice. For ordinary small fragment vectors, the linear integration efficiency is higher than that of circular ones. Therefore, the constructed expression vectors must be enzymatically cut into linear shapes for microinjection. However, due to its large structure, the large-fragment BAC vector is prone to breakage during the recovery process after linearization. If it is not recovered, the same high transgenic efficiency can be obtained, whic...
Embodiment 3
[0079] Example 3 Preparation of Highly Expressed Human Lysozyme Transgenic Cattle
[0080] 3.1 Establishment of bovine fetal fibroblast cell line
[0081] Bovine fetal fibroblasts were established by trypsinization. The specific method is as follows: a. Slaughter the 42-day cow, take out the placenta and put it in DMEM medium, and return to the laboratory after 12 hours. b. Remove the fetal head, limbs, viscera and cartilage tissue in a cell culture dish containing 5×DPBS, put the remaining tissue into a new 10cm cell culture dish, and cut it as much as possible with ophthalmic scissors. c. Add 1ml of complete medium, transfer the tissue fragments and medium to a 15ml centrifuge tube with a 1ml tip of a scissors tip, add 7ml of DPBS, pipette a few times, and discard the tissue after the tissue pieces sink. supernatant, and repeat the wash once. d. Add 10ml of 0.25% trypsin to a 15ml centrifuge tube, then digest in a 37°C water bath for 30min. e. Carefully pipette the super...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com