Method for producing metaldehyde
A production method, metaldehyde technology, applied in the direction of organic chemistry, can solve the problems of high energy consumption and low product yield, and achieve the effects of less dosage, short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
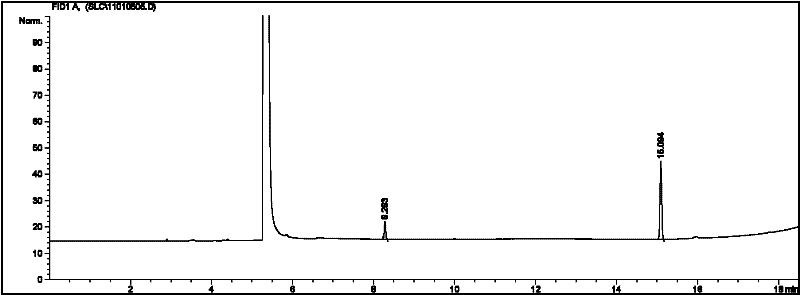
[0028] Acetaldehyde (25mL) was cooled to 0°C, 2mL ether and 0.5mL pyridine were added, the temperature of the mixture was lowered to -15°C, and 0.4mL thionyl chloride (SOCl 2 ), control the catalyst addition rate, and ensure that the temperature of the reaction mixture does not exceed 0°C. After the catalyst is added, stir vigorously at -15°C for 30 minutes, then filter, wash the solid with 20mL of ether, and dry it in vacuum to obtain a white crystal Metaldehyde 1.88g, yield 9.6%. Gas chromatography test results are attached figure 1 , peak time: 8.283min, 15.094min Area% 15.131, 84.869.
Embodiment 2
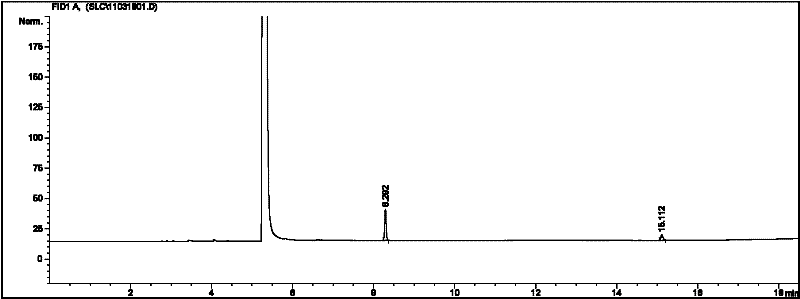
[0030] Acetaldehyde (25mL) was cooled to 0°C, 2mL of ether and 0.4mL of pyridine were added, the temperature of the mixture was lowered to -15°C, and 0.4mL of phosphine pentachloride (PCl 5 ), control the catalyst addition rate, and ensure that the temperature of the reaction mixture does not exceed 0°C. After the catalyst is added, stir vigorously at -15°C for 30 minutes, then filter, wash the solid with 20mL of ether, and dry it in vacuum to obtain a white crystal Metaldehyde 0.9g, yield 4.6%. Gas chromatography test results are attached figure 2 , peak time: 8.292min, 15.112 min Area%, 79.112, 20.878.
Embodiment 3
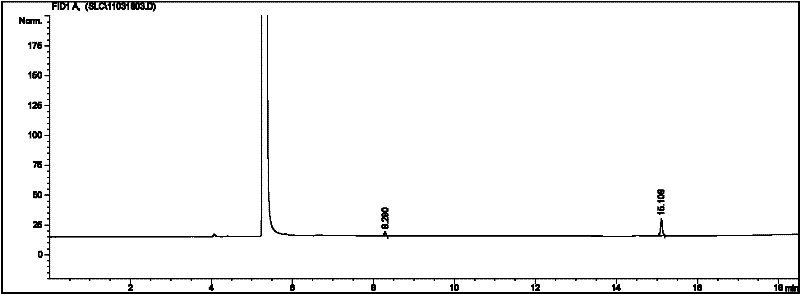
[0032] Acetaldehyde (25mL) was cooled to 0°C, 2mL ether and 0.4mL pyridine were added, the temperature of the mixture was lowered to -15°C, and 0.4mL sulfuryl chloride (SO 2 Cl 2 ), control the catalyst addition rate, and ensure that the temperature of the reaction mixture does not exceed 0°C. After the catalyst is added, stir vigorously at -5°C for 30 minutes, then filter, wash the solid with 20mL ether, and vacuum dry to obtain a white crystal Metaldehyde 1.48g, yield 7.6%. Gas chromatography test results are attached image 3 , peak time: 8.292min, 15.108 min Area%, 16.250, 83.750.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com