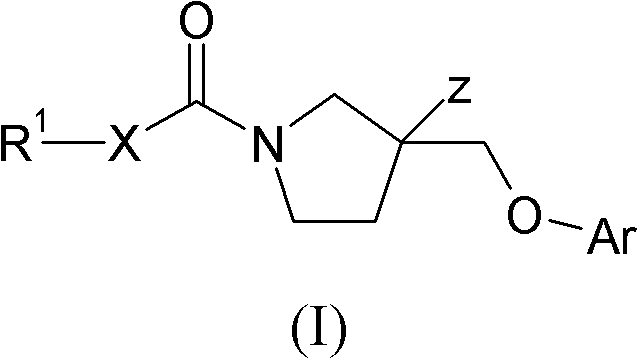
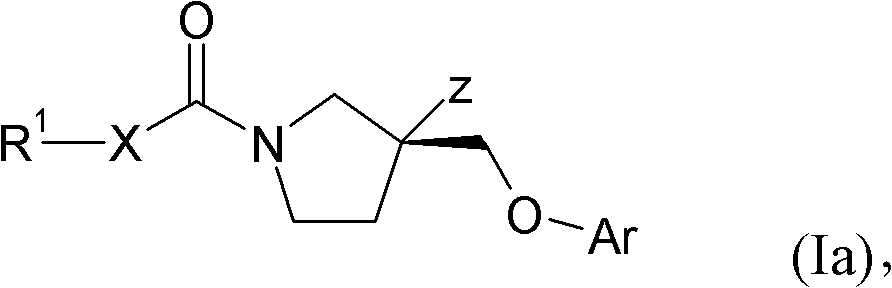
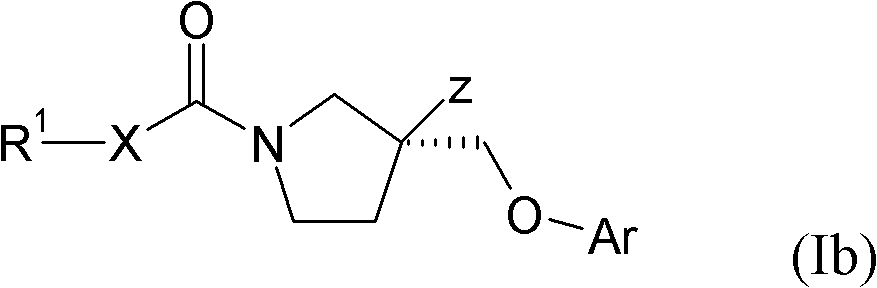
Pyrrolidines
A compound and hydrate technology, applied in the direction of effective components of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems of insufficient efficacy and selectivity in medical treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0366] Example 1: 3-(4'-Cyano-biphenyl-4-yloxymethyl)-1-(4-methoxy-benzoyl)-pyrrolidine-3-carboxylic acid (enantiomer body 1)
[0367]
[0368] Sodium trimethylsilanolate (72.1 g) and water (7.72 ml, 428.6 mmol) were added to the stirred 3-(4'-cyano-biphenyl-4-yloxymethyl)-1-(4 -Methoxy-benzoyl)-pyrrolidine-3-carboxylic acid ethyl ester (see Preparation 28a) (207.7 g, 428.6 mmol) in acetonitrile (2090 mL). The resulting mixture was stirred at room temperature (RT) for 18 hours. The reaction mixture was filtered and the solid was partitioned between ethyl acetate (2000 mL) and aqueous hydrogen chloride (2000 mL, 2M). The organic layer was washed with water (1000 mL), then concentrated under reduced pressure to give a pale orange foam. Recrystallization twice sequentially from ethyl acetate (700 mL) and isopropanol (700 mL) afforded the title compound (107.2 g, 55%) as a white solid.
[0369] 1H NMR (400MHz, DMSO d-6) δppm 1.89-2.05(m,1H), 2.15-2.19(m,1H), 3.40-3.53(m,3H)...
Embodiment 2
[0372] Example 2: 3-(4'-Cyano-biphenyl-4-yloxymethyl)-1-(4-fluoro-benzoyl)-pyrrolidine-3-carboxylic acid (Enantiomer 1 )
[0373]
[0374] Lithium hydroxide (16 mg, 0.67 mmol) was added to stirred 3-(4'-cyano-biphenyl-4-yloxymethyl)-1-(4-fluoro-benzoyl)-pyrrolidine - Ethyl 3-carboxylate (see Preparation 29, peak 2) (105 mg, 0.22 mmol) in ethanol (1 mL) and water (1 mL). The resulting mixture was stirred at room temperature for 5 hours. The mixture was concentrated under reduced pressure to remove ethanol and the residue was partitioned between ethyl acetate (5 mL) and 2M aqueous HCl (5 mL). The organic layer was washed with brine (5 mL), dried over magnesium sulfate and concentrated under reduced pressure to give a white solid. Recrystallization from isopropanol (2.5 mL) gave the title compound (76 mg, 77%) as a white solid.
[0375] LCMSRt 3.06 min, ES m / z 445 [MH] +
[0376] This method is hereinafter referred to as hydrolysis method B.
Embodiment 3
[0377] Example 3: 3-(4-(5-Cyanopyridin-2-yl)phenyloxymethyl)-1-(2-methoxybenzoyl)-pyrrolidine-3-carboxylic acid (enantio Isomer 1)
[0378]
[0379] Potassium trimethylsilanolate (14 mg, 0.10 mmol) was added to the stirred 3-[4-(5-cyano-pyridin-2-yl)-phenoxymethyl]-1-(2-methoxy yl-benzoyl)-pyrrolidine-3-carboxylic acid ethyl ester (see Preparation 43, peak 1) (40 mg, 0.08 mmol) in tetrahydrofuran (5 mL). The resulting mixture was stirred at room temperature for 18 hours. The reaction mixture was concentrated under reduced pressure to remove ethanol and the residue was partitioned between ethyl acetate (5 mL) and 2M aqueous HCl (5 mL). The organic layer was washed with brine (5 mL), then concentrated under reduced pressure to give a colorless gum. The residue was purified by silica gel column chromatography, which was successively eluted with ethyl acetate and methanolic ammonia solution to give the title compound (18 mg, 48%) as a white solid.
[0380] LCMSRt 2.79 min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com