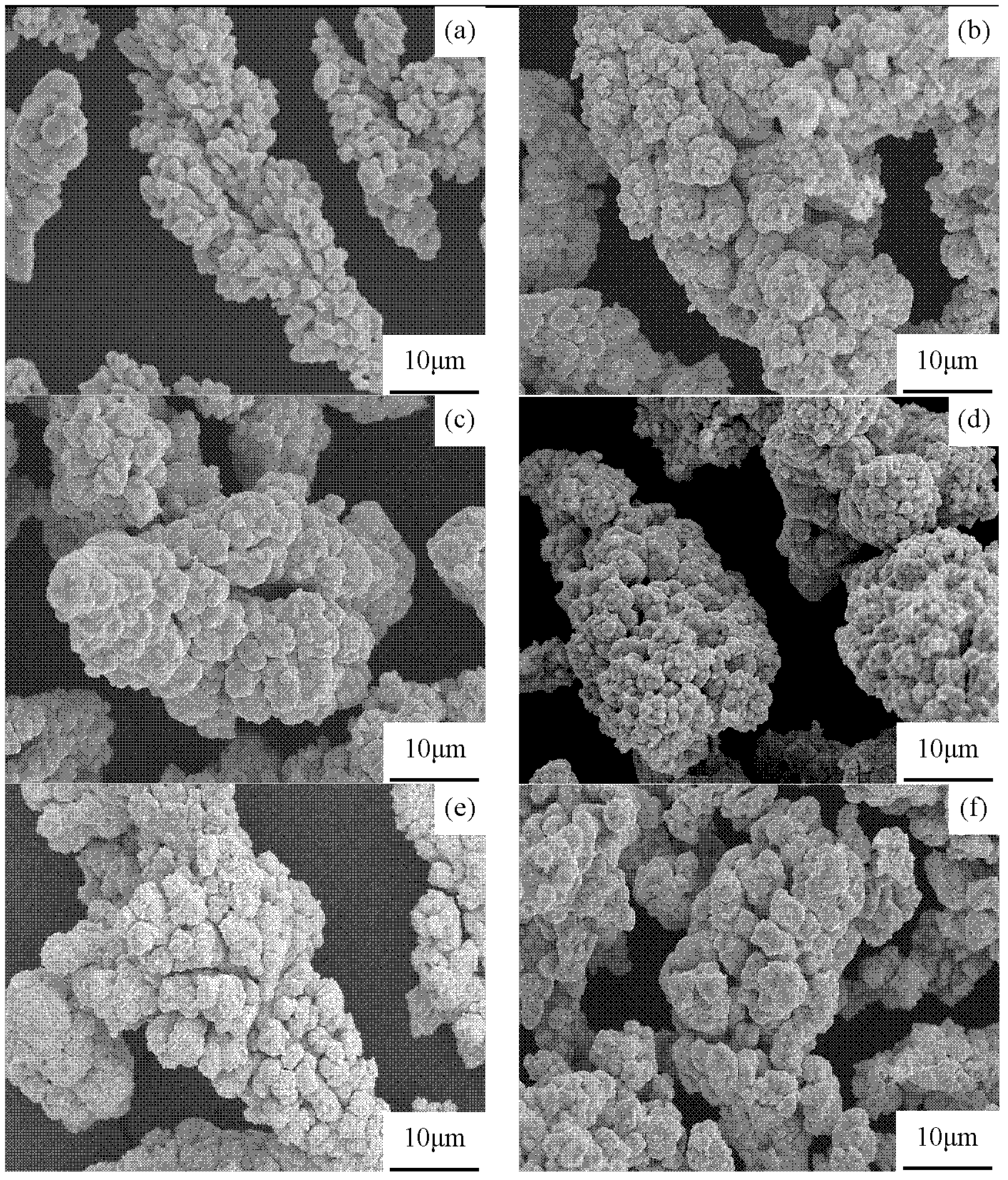Method for plating silver on surface of dendritic copper powder
A dendritic silver and copper powder technology, which is applied in liquid chemical plating, coating, metal material coating process, etc., can solve the problem of many chemical raw materials, incomplete coating of silver-coated copper powder, and complicated copper powder pretreatment process and other issues to achieve the effect of process safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 5g of copper powder, add it to 200ml of dilute sulfuric acid with a weight ratio of 5%, and pickle it for 25 minutes under stirring conditions to remove oxides on the surface of the copper powder. After washing with deionized water three times, filter the water and set aside. Get 2.25g silver nitrate (Cu, AgNO 3 The molar ratio is 1:0.37) into 50ml of deionized water, add an appropriate amount of ammonia water until the solution becomes transparent; then weigh 0.25g of NaOH and dissolve it in 10ml of deionized water, then add the NaOH solution to the silver nitrate solution, the solution becomes cloudy , turn black, add ammonia water dropwise until the solution is clear. In 100ml deionized water, add 2.25g glucose (at this time AgNO 3 , glucose molar ratio is 1:0.94) and 0.3g tartaric acid, after stirring evenly, heat and boil for 10min, and obtain a reducing solution after cooling. Add the copper powder after pickling to the reducing agent solution, and add the ...
Embodiment 2
[0029] Take 10g of copper powder, add it to 400ml of dilute sulfuric acid with a weight ratio of 5%, and pickle it for 30 minutes under stirring conditions to remove oxides on the surface of the copper powder. After washing with deionized water three times, filter the water and set aside. Take 5g silver nitrate (Cu, AgNO 3 The molar ratio is 1:0.19) into 50ml of deionized water, add an appropriate amount of ammonia until the solution becomes transparent; then weigh 0.6g of NaOH and dissolve it in 10ml of deionized water, then add the NaOH solution to the silver nitrate solution, the solution becomes cloudy , turn black, add ammonia water dropwise until the solution is clear. In 200ml deionized water, add 5.5g glucose (at this time AgNO 3 , glucose molar ratio is 1: 1.04) and 0.5g tartaric acid, after stirring evenly, heat and boil for 10min, and obtain a reducing solution after cooling. Add the copper powder after pickling to the reducing agent solution, and add the main sal...
Embodiment 3
[0031] Take 50g of copper powder, add it to 2L dilute sulfuric acid with a weight ratio of 6%, pickle it for 30min under stirring condition, remove the oxide on the surface of copper powder, wash it with deionized water three times, filter the water and set aside. Get 30g silver nitrate (Cu, AgNO 3 The molar ratio is 1:0.22) into 200ml of deionized water, add an appropriate amount of ammonia water until the solution becomes transparent; then weigh 2.5g of NaOH and dissolve it in 50ml of deionized water, then add the NaOH solution to the silver nitrate solution, the solution becomes cloudy , turn black, add ammonia water dropwise until the solution is clear. Add 20g of glucose to 3L of deionized water (at this time AgNO 3 , glucose molar ratio is 1:0.63) and 4g of tartaric acid, after stirring evenly, heat and boil for 10min, and obtain a reducing solution after cooling. Add the copper powder after pickling to the reducing agent solution, and add the main salt according to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com