Inorganic semiconductor nanometer material and preparation method thereof
A technology of inorganic semiconductors and nanomaterials, applied in the field of nanomaterials, can solve the problem of small size dependence, and achieve the effect of simple method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] [Example 1]: Preparation of chiral cadmium telluride nanoparticles
[0036] 1) Dissolve 0.5g of cadmium perchlorate (ie, cationic complexing agent) and 0.1g of D-cysteine and L-cysteine (ie, chiral stabilizer) in water, and adjust the pH value to 11 (Concrete method can refer to J.Phys.Chem.B 2002,106,7177-7185);
[0037] 2) Then introduce hydrogen telluride gas (i.e. anion complexing agent) under an inert atmosphere, heat at 80-100°C to grow CdTe, and then obtain D-cysteine-stabilized cadmium telluride nanoparticles and L-cysteine stabilized cadmium telluride nanoparticles.
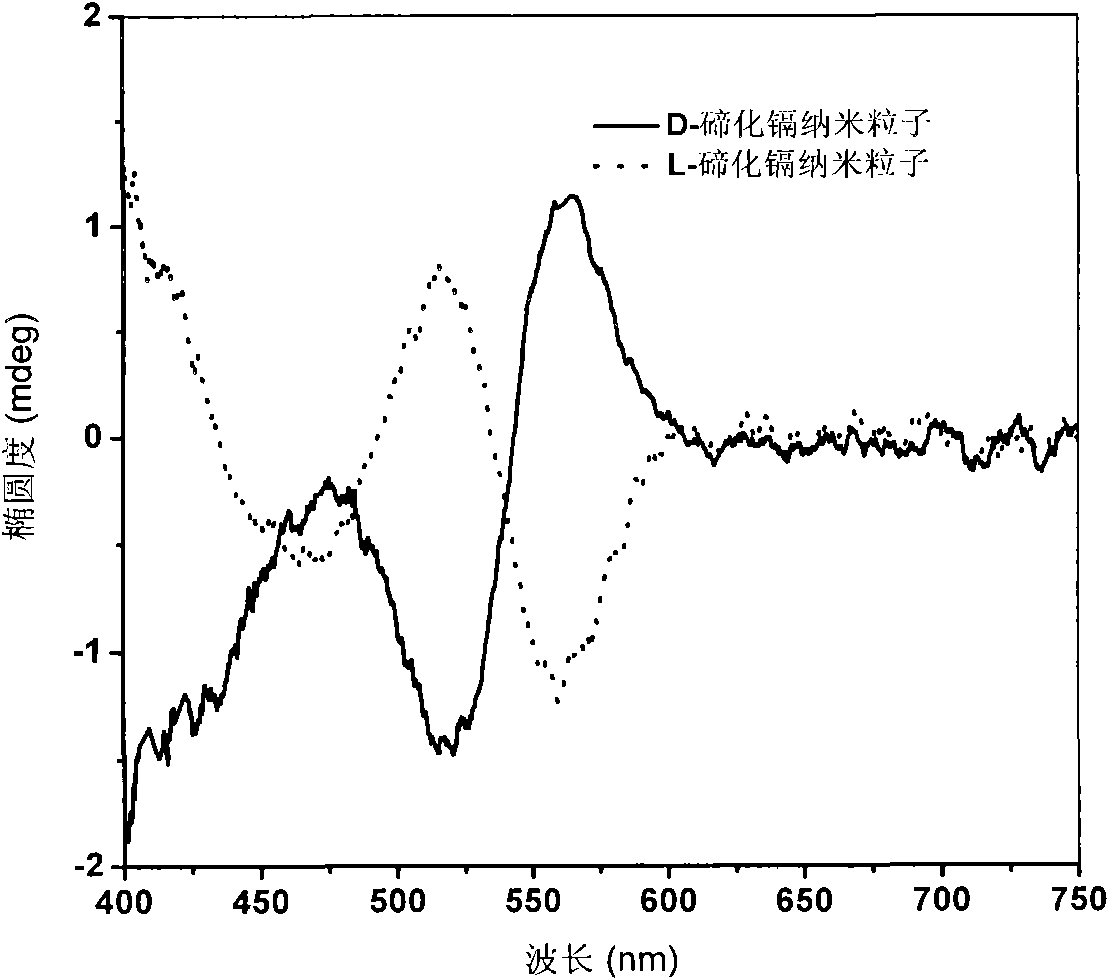
[0038] The chiral characteristics of materials are characterized by measuring the difference in absorption rate of chiral substances for left-handed and right-handed polarized light by circular dichroism spectroscopy. figure 1 Circular dichroism spectra of D-cadmium telluride and L-cadmium telluride chiral nanoparticles. From this figure, it can be seen that the circular dichroism spectra...
Embodiment 2
[0039] [Example 2]: Preparation of chiral tellurium cadmium sulfide alloy nanoparticles
[0040] 1) Weigh 0.5g cadmium perchlorate (ie, cationic complexing agent) and 0.6g L-glutathione (ie, chiral stabilizer), dissolve the above two substances in water, and adjust the pH value to 11.3;
[0041] 2) Then introduce hydrogen telluride gas (that is, the anion complexing agent) under an inert atmosphere, and heat at 100° C. to obtain chiral cadmium telluride sulfide alloy nanoparticles.
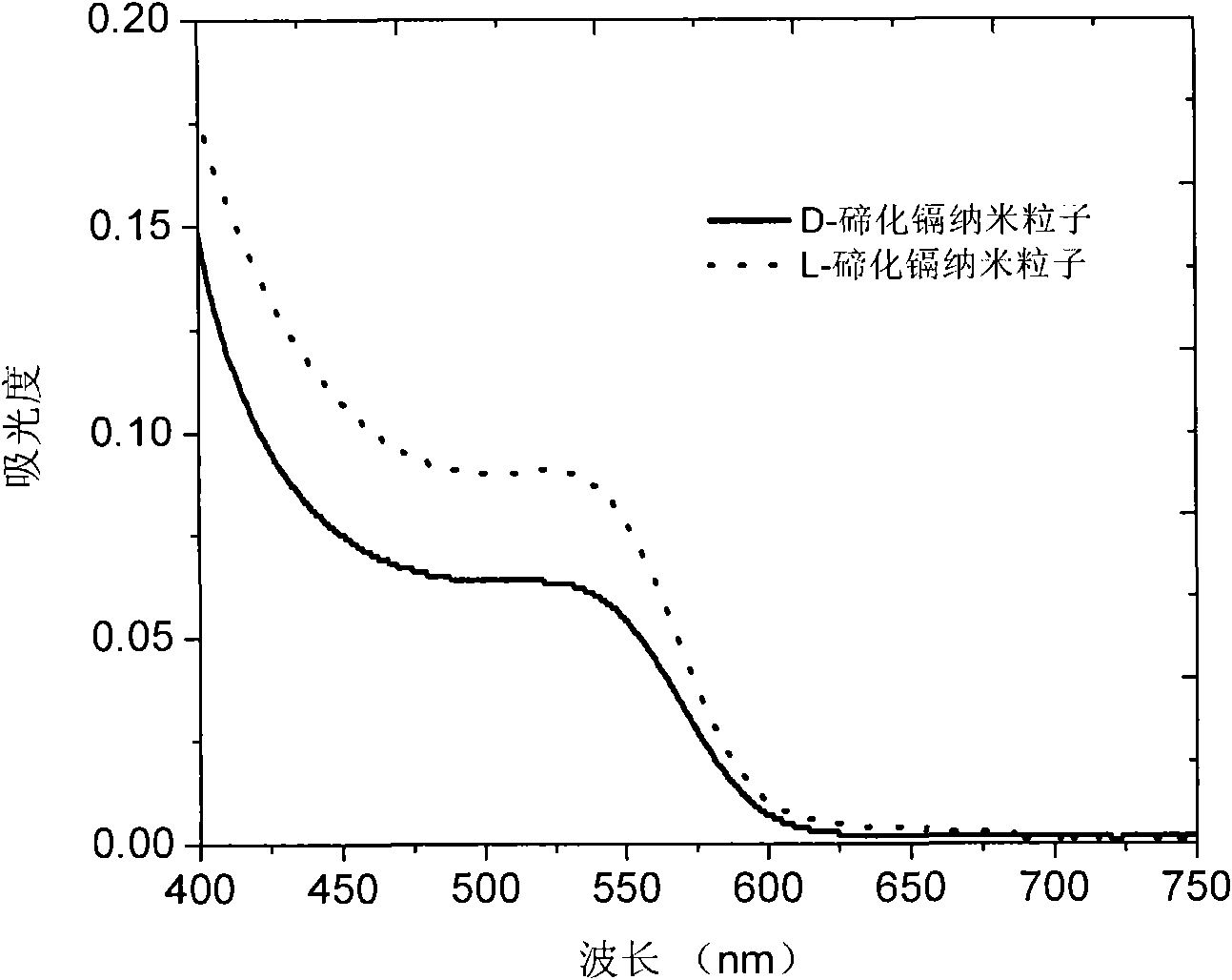
[0042] Figure 5 It is the circular dichroism spectrum and the ultraviolet-visible spectrum of chiral cadmium telluride sulfide. The two absorption peaks of the ultraviolet-visible spectrum are attributed to CdS and CdTe, and both of them have optical absorption in the circular dichroism spectrum. Image 6 The XRD pattern of the product reflects the structure characteristics of sphalerite of cadmium telluride sulfide. combine Figure 5 , Image 6 It can be seen that the obtained nanoparticles ...
Embodiment 3
[0043] [Example 3]: Preparation of chiral cadmium telluride nanorods
[0044] 1) First weigh 0.5g cadmium perchlorate (ie, cationic complexing agent), and dissolve them in water with 0.05g of D-cysteine and 0.05g of L-cysteine (ie, chiral stabilizer) , and add 0.1ml thioglycolic acid as an auxiliary complexing agent to adjust the pH value to 11;
[0045] 2) Then introduce hydrogen telluride gas under an inert atmosphere and heat at 100° C. to obtain (D, L) cysteine-stabilized cadmium telluride nanorods.
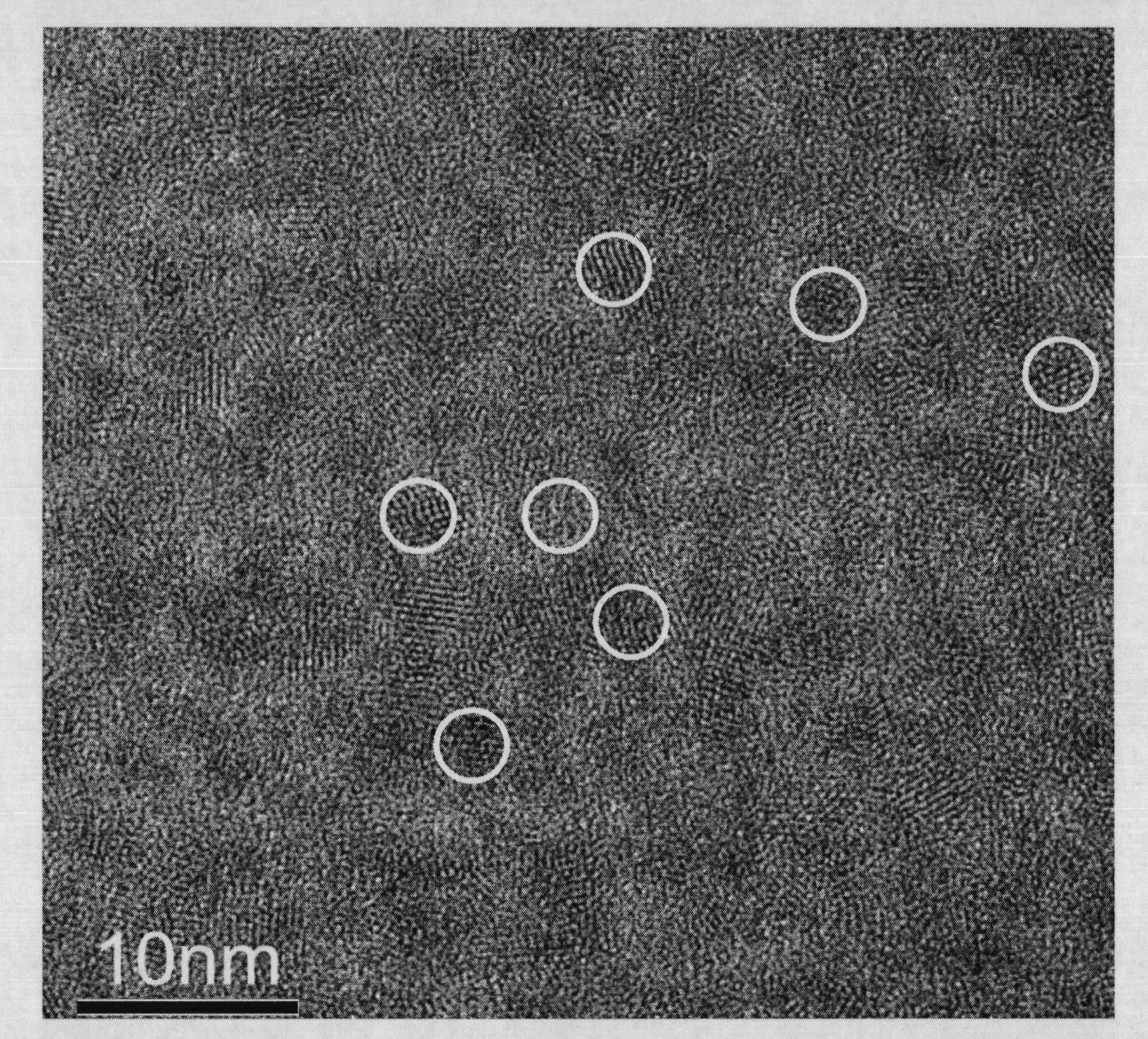
[0046] Figure 7 It is a TEM photo of cadmium telluride chiral nanorods, from which it can be seen that the prepared cadmium telluride nanorods have a width of about 5 nm and a length of more than 100 nm. Figure 8 It is the circular dichroism spectrum of cadmium telluride chiral nanorods. From this figure, it can be seen that the circular dichroism spectra of (D- and L-) cadmium telluride nanorods are mirror-symmetrical and have obvious chiral characteristics. In this...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com